
Diagnostic accuracy of 13N-ammonia myocardial perfusion imaging with PET-CT in the detection of coronary artery disease
Introduction
Positron emission tomography (PET) myocardial perfusion (MPI) has several advantages over single-photon emission computed tomography (SPECT) MPI. For one, PET-MPI offers higher spatial resolution as PET radiotracers have higher energy than SPECT radiotracers. In addition, PET cameras do not require physical collimation and therefore offer better count sensitivity, better temporal resolution, lower radiation dose and more advanced technology for measurement of myocardial flow reserve (MFR). Accordingly, PET-MPI has higher sensitivity and specificity (1,2), as well as improved image resolution and intrinsic attenuation correction (3). The clinical superiority of PET-MPI was recognized in a joint statement of the American Society of Nuclear Cardiology and Society of Nuclear Medicine and Molecular Imaging (4). Currently, rubidium-82 (Rb-82) and 13N-ammonia are the only two FDA-approved radiopharmaceuticals used for myocardial perfusion PET. PET-MPI is being increasingly used due to availability of generator-produced Rb-82 at imaging centers that lack a cyclotron. However, Rb-82 has relatively poor resolution. 13N-ammonia has a high extraction rate, as a result of which it is simpler to quantify it; in addition, it provides better image quality than Rb-82 (5), There are several published reviews and meta-analyses that have reported the high sensitivity and specificity of PET-MPI (6,7). However, most of these studies used Rb-82Jeny (8-13), and there is very little information on the diagnostic accuracy of 13N-ammonia as a PET myocardial perfusion radiotracer (14-16). The most exciting literature on the diagnostic accuracy of 13N-ammonia PET-CT myocardial perfusion was published before 1990 and included only a small number of patients. Therefore, there is a need for more data on the diagnostic accuracy of 13N-ammonia PET-MPI. The main aim of this study was to fill in this information gap by investigating the diagnostic accuracy of 13N-ammonia PET-MPI and comparing it with the gold standard, invasive coronary angiography (ICA).
Methods
Study patients
The study participants included 383 patients who underwent PET-MPI based on their clinical indications between July 2014 and January 2018. Eligible patients were identified retrospectively from the nuclear medicine and PET center data base. Patients were excluded if they had undergone treatment for coronary artery disease (CAD), such as previous percutaneous coronary intervention (PCI), CA (for which the findings were abnormal), previous coronary artery bypass grafting (CABG), or previous MPI (for which the findings were abnormal). In addition, 4 patients were excluded because their PET scan could not be used for diagnosis, because of, for example, excessive uncorrected misregistration between the emission and transmission scan and extensive lung uptake. All patients with abnormal PET findings and selected patients with normal PET findings were referred for ICA within 60 days from the PET scan. The study was approved by our hospital’s institutional review board.
13N-ammonia PET-MPI
PET was performed with the GE discovery (CVT or 710) PET-CT scanner (GE Medical System, Milwaukee, Wisconsin). Rest data were acquired immediately after injection of 700–900 MBQ of 13N-ammonia into a peripheral vein. Stress data were acquired approximately 4 halftimes (40 min) after the acquisition of the rest data. Stress was induced through adenosine infusion; another 700–900 MBQ of 13N-ammonia was injected 2 min after the adenosine injection, after which a transmission scan was obtained for attenuation correction as previously reported (17). The PET findings were interpreted by two experienced nuclear medicine physicians, and disagreements were resolved by consensus. The 17-segment model and semiquantification scoring system (0 = normal, 1 = mildly abnormal, 2 = moderately abnormal, and 4 = completely abnormal) recommended by the American Society of Nuclear Cardiology (18) were employed. Images were considered to be normal when the summed stress score (SSS) was less than 4, mildly abnormal when the SSS was 4 were employed. Images were considered to be normal when the summed stress score (SSS) was les (19) (Figures 1,2).
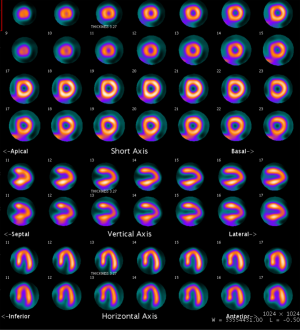
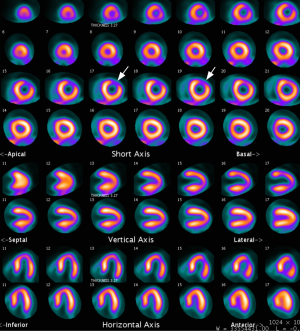
ICA
Conventional ICA was performed within 60 days after PET-MPI. The coronary arteries were divided into segments, according to the method of the American Heart Association, for Coronary Tomography Coronary Angiography (CTCA) analysis (20). The angiograms were analyzed by two interventional cardiologists who were blinded to the CTCA results. Stenosis was considered to be significant if lumen reduction was more than 50%.
Coronary calcification scoring
Prospective ECG gating and dose modulation were utilized to minimize the radiation dose for 64-slice PET-CT scanners. CT was acquired under the following conditions: voltage, 120 kvp; current, 100 mA; collimation, 0.6 mm; total acquisition time, 10 s; radiation dose, approximately 1 mSv. All CT images were reconstructed with 3-mm thickness and a medium smooth filter. Coronary calcium scoring was then performed using Agatston methods (21).
Statistical analysis
Descriptive statistics were presented as means and standard deviations for data measured on the continuous scale, and as proportions for data measured on the categorical scale. The significance of the association between pairs of categorical variables was tested using Pearson’s chi-square test. The significance of the difference between group means was tested using one-way ANOVA. Multiple linear regressions for continuous outcomes and multiple logistic regression for dichotomous outcomes were used to evaluate the joint effect of potential risk factors. The type I error rate was set at 5%. The commercial program SPSS (version 20) was used for data analysis.
Results
The total study population was 383, of which 215 (56%) were male and 168 (44%) were female. The mean age was 64±11 years, and the mean coronary artery calcium score (CACS) was 330±589. The majority of patients were obese or overweight, and the mean body mass index (BMI) was 32±7 kg/m2. Risk factors for CAD were prevalent in all the patients, especially hypertension and diabetes mellitus. The patient characteristics are presented in Table 1.
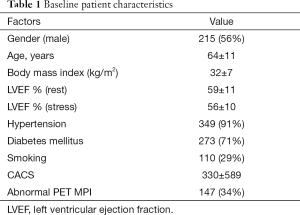
Full table
Diagnostic accuracy of PET-MPI
Out of the total 383 patients, 236 had normal PET-MPI findings, and ICA was performed in only 68 of these patients. Out of these, 52 patients had normal ICA findings (true negative) and 16 patients had abnormal ICA findings (false negative). Of the 147 patients who had abnormal PET-MPI findings, ICA was not performed in only 2 patients. In the remaining 145 patients who underwent ICA, 6 had normal ICA findings (false positive) and 139 had abnormal ICA findings (true positive). The sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of PET-MPI was 90%, 90%, 96%, 76%, and 80% respectively.
Association between CAD risk factors and PET-MPI, ICA, and CACS
Multiple linear regression analysis showed that only age, diabetes, and gender were predictors of CAC (P=0.000, 0.049, and 0.016 respectively). Further, multiple linear regression analysis showed that age and smoking were the only predictors of PET-MPI (P=0.022 and 0.010 respectively). Multiple logistic regression analysis also showed that only age and gender were predictors of ICA (P=0.021 and 0.013 respectively) (Table 2).
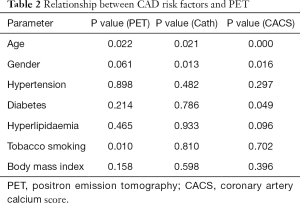
Full table
Relationship between CACS and PET-MPI
The number of patients who had a CACS of 0 was 137 (36%) (Group 1); CACS of 1–300, 127 (33%) (Group 2); and CACS >300, 119 (31%) (Group 3). A strong correlation was found between CACS subgroup and PET-MPI and ICA values (P=0.000 for both correlations) (Table 3).
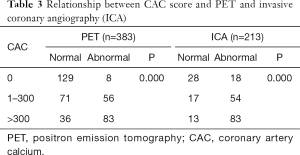
Full table
Discussion
The main finding of our study was that 13N-ammonia PET-MPI has high diagnostic accuracy for the detection of coronary artery stenosis of more than 50%; in addition, it had a sensitivity of 90%, specificity of 90%, positive predictive value of 96%, negative predictive value of 76%, and overall diagnostic accuracy of 80%. Notably, the diagnostic accuracy was high in both male and female patients as well as overweight and obese patients (mean BMI, 32±7 kg/m2).
Although the first report on the superior diagnostic accuracy of 13N-ammonia PET-MPI by Tamaki was published approximately three decades ago (14), very few studies have followed it. Further, the few studies have reported inconsistent results and used a small patient group. The most exciting finding on the diagnostic accuracy of PET-MPI was obtained with Rb-82. The commercial availability of Rb-82 generators is considered to be a key element for the widespread application of Rb-82 myocardial perfusion PET. In contrast, 13N-ammonia requires an in-house or closely located cyclotron. A review article by Di Calri et al. in 2007 summarized 9 published studies on the diagnostic accuracy of PET-MPI for detecting more than 50% coronary artery stenosis (22). The overall sensitivity was 90%; specificity, 89%; positive predictive value, 94%; and negative predictive value, 73% (22). Interestingly, 6 out of these 9 studies were performed with Rb-82, 2 were performed with ammonia and Rb-82, and only 1 was performed with 13N-ammonia. A study by Husmann et al. reported that the sensitivity of 13N-ammonia PET-MPI was 97% and its specificity was 91% (23). Such a high diagnostic accuracy was probably obtained because most of the study population had CAD: 49 patients had been diagnosed with CAD and only 21 patients were suspected of having CAD (23).
Currently, there are no studies that compare the diagnostic accuracy of ammonia PET with SPECT, the most commonly used technique to assess myocardial perfusion. However, several studies have compared the diagnostic accuracy of PET-MPI with SPECT-MPI in the same or matched patients. For example, Bateman et al. compared Rb-82 PET and TC-99m sestamibi in two matched patients and found that the overall diagnostic accuracy with a 50% (87% vs. 71%) or 70% (89% vs. 79%) angiographic threshold was higher for PET than for SPECT (9). In another study by Stewart et al. in which PET was compared with SPECT in 81 patients, PET was found to have higher specificity (96%) than SPECT (53%) (11). Sampson et al. reported that Rb-82 PET-CT MPI had a sensitivity of 93% and specificity of 83% (8). In their study, obstructive CAD was considered if ICA showed more than 70% stenosis. In the present study, 50% or more stenosis was considered to be indicative of obstructive CAD. This may explain the slight difference in the sensitivity and specificity between our study and their study.
The role of 13N-ammonia in predicting functional outcome after coronary revascularization has been studied by Duvernoy et al.: preoperative relative uptake greater than 80% and less than 40% was found to have excellent predictive value for functional outcome (24). Further, Sand et al. found that MPI with 13N-ammonia and Tc-99m sestamibi had similar results in patients with severe left ventricular function (25). Only one study has reported the long-term outcome of 13N-ammonia: this study by Fiechter et al. reported that perfusion findings for 13N-ammonia are strong predictors of long-term outcome in 612 patients for 5.7±2.5 years (26).
Modern PET-CT cameras allow CACS to be acquired as part of the myocardial perfusion imaging protocol. CACS is the most common parameter used for assessing subclinical CAD (27). In our study, using CACS, we found that subclinical CAD was present in 45% of patients with suspected CAD with normal PET-MPI findings. This finding is important as a previous report had shown that adding the CACS to the Framingham risk score (FRS), especially in the case of patients with intermediate FRS values, can improve prognosis (28,29). Our results also reaffirm the findings of Bybee et al., who calculated CACS in 760 patients with no history of CAD and normal Rb-82 PET-MPI stress perfusion findings: in their study, 64% of patients with normal PET-MPI had subclinical CAD, as indicated by the CACS, based on which the authors concluded that subclinical CAD is common in patients without known CAD and normal PET-MPI findings (30). The association of CACS and normal SPECT-MPI has been previously studied by our group in 207 patients with normal SPECT MPI, in which the CACS was zero in 45% and abnormal in 55% (31). Of those with abnormal CACS, 43% had a CACS of 1–300, and 12% had a CACS of more than 300. Furthermore, there was a strong association between CACS and age, male gender, and diabetes (31). In addition, there was a strong correlation between CACS and ICA (P=0.000). Almost 50% of patients with normal ICA findings had a CACS of 0, but obstructive CAD was diagnosed in symptomatic patients based on the CACS alone. In our current study, 12% of patients with a CACS value of 0 had abnormal ICA findings. This high percentage compared to previous reports can be explained as follows: first, this group of patients was at high risk and symptomatic, and second, there is a sampling bias because most patients with normal CACS were not referred for ICA. Kim et al. also reported that a CACS value of 0 cannot be used to exclude obstructive CAD in symptomatic patients who were referred for CTCA; the prevalence of obstructive CAD in symptomatic patients with a CACS of 0 was 7.4% in men and 2% in women. Moreover, in their study, it was noted that adverse cardiac events are not negligible in symptomatic patients with a CACS of 0 (32).
Study limitations
Quantitative myocardial blood flow (CFR) was not determined in the majority of patients. Subsequently, it was not included in the data analysis. CFR can improve the diagnostic accuracy of myocardial perfusion, as it can be used to detect balanced ischemia and left main disease that could be missed with static perfusion images only. The severity of obstructive CAD was determined visually rather than quantitatively; however, this is the common clinical practice. Finally, this is a retrospective study that was conducted at a single, tertiary care center and the possibility of a referral bias cannot be excluded.
Conclusions
13N-ammonia PET-CT myocardial perfusion offers very high sensitivity, specificity, and overall diagnostic accuracy for the detection of obstructive CAD. This high diagnostic accuracy is observed in male and female, as well as overweight and obese patients. Adding CACS as a diagnostic factor in patients with unknown CAD and PET-MPI may help in the detection of subclinical CAD, guiding CAD management and prevention, and providing prognostic information.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was waived by the institutional review board for this retrospective study.
References
- Slart RH, Bax JJ, van Veldhuisen DJ, et al. Imaging techniques in nuclear cardiology for the assessment of myocardial viability. Int J Cardiovasc Imaging 2006;22:63-80. [Crossref] [PubMed]
- Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation 2003;108:1404-18. [Crossref] [PubMed]
- Koepfli P, Hany TF, Wyss CA, et al. CT attenuation correction for myocardial perfusion quantification using a PET/CT hybrid scanner. J Nucl Med 2004;45:537-42. [PubMed]
- Bateman TM, Dilsizian V, Beanlands RS, et al. American Society of Nuclear Cardiology and Society of Nuclear Medicine and Molecular Imaging Joint Position Statement on the Clinical Indications for Myocardial Perfusion PET. J Nucl Med 2016;57:1654-6. [Crossref] [PubMed]
- Di Carli MF, Murthy VL. Cardiac PET/CT for the evaluation of known or suspected coronary artery disease. Radiographics 2011;31:1239-54. [Crossref] [PubMed]
- Di Carli MF, Dorbala S, Meserve J, et al. Clinical Myocardial Perfusion PET/CT. J Nucl Med 2007;48:783-93. [Crossref] [PubMed]
- Machac J. Cardiac positron emission tomography imaging. Semin Nucl Med 2005;35:17-36. [Crossref] [PubMed]
- Sampson UK, Dorbala S, Limaye A, et al. Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol 2007;49:1052-8. [Crossref] [PubMed]
- Bateman TM, Heller GV, McGhie AI, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol 2006;13:24-33. [Crossref] [PubMed]
- Grover-McKay M, Ratib O, Schwaiger M, et al. Detection of coronary artery disease with positron emission tomography and rubidium 82. Am Heart J 1992;123:646-52. [Crossref] [PubMed]
- Stewart RE, Schwaiger M, Molina E, et al. Comparison of rubidium-82 positron emission tomography and thallium-201 SPECT imaging for detection of coronary artery disease. Am J Cardiol 1991;67:1303-10. [Crossref] [PubMed]
- Marwick TH, Nemec JJ, Stewart WJ, et al. Diagnosis of coronary artery disease using exercise echocardiography and positron emission tomography: comparison and analysis of discrepant results. J Am Soc Echocardiogr 1992;5:231-8. [Crossref] [PubMed]
- Go RT, Marwick TH, MacIntyre WJ, et al. A prospective comparison of rubidium-82 PET and thallium-201 SPECT myocardial perfusion imaging utilizing a single dipyridamole stress in the diagnosis of coronary artery disease. J Nucl Med 1990;31:1899-905. [PubMed]
- Tamaki N, Yonekura Y, Senda M, et al. Value and limitation of stress thallium-201 single photon emission computed tomography: comparison with nitrogen-13 ammonia positron tomography. J Nucl Med 1988;29:1181-8. [PubMed]
- Demer LL, Gould KL, Goldstein RA, et al. Assessment of coronary artery disease severity by positron emission tomography. Comparison with quantitative arteriography in 193 patients. Circulation 1989;79:825-35. [Crossref] [PubMed]
- Gould KL, Goldstein RA, Mullani NA, et al. Noninvasive assessment of coronary stenoses by myocardial perfusion imaging during pharmacologic coronary vasodilation. VIII. Clinical feasibility of positron cardiac imaging without a cyclotron using generator-produced rubidium-82. J Am Coll Cardiol 1986;7:775-89. [Crossref] [PubMed]
- Herzog BA, Husmann L, Valenta I, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 2009;54:150-6. [Crossref] [PubMed]
- Machac J, Bacharach SL, Bateman TM, et al. Positron emission tomography myocardial perfusion and glucose metabolism imaging. J Nucl Cardiol 2006;13:e121-51. [Crossref] [PubMed]
- Chow BJ, Beanlands RS, Lee A, et al. Treadmill exercise produces larger perfusion defects than dipyridamole stress N-13 ammonia positron emission tomography. J Am Coll Cardiol 2006;47:411-6. [Crossref] [PubMed]
- Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975;51:5-40. [Crossref] [PubMed]
- Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827-32. [Crossref] [PubMed]
- Di Carli MF, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation 2007;115:1464-80. [Crossref] [PubMed]
- Husmann L, Wiegand M, Valenta I, et al. Diagnostic accuracy of myocardial perfusion imaging with single photon emission computed tomography and positron emission tomography: a comparison with coronary angiography. Int J Cardiovasc Imaging 2008;24:511-8. [Crossref] [PubMed]
- Duvernoy CS, vom Dahl J, Laubenbacher C, et al. The role of nitrogen 13 ammonia positron emission tomography in predicting functional outcome after coronary revascularization. J Nucl Cardiol 1995;2:499-506. [Crossref] [PubMed]
- Sand NP, Bottcher M, Madsen MM, et al. Evaluation of regional myocardial perfusion in patients with severe left ventricular dysfunction: comparison of 13N-ammonia PET and 99mTc sestamibi SPECT. J Nucl Cardiol 1998;5:4-13. [Crossref] [PubMed]
- Fiechter M, Gebhard C, Ghadri JR, et al. Myocardial perfusion imaging with 13N-ammonia PET is a strong predictor for outcome. Int J Cardiol 2013;167:1023-6. [Crossref] [PubMed]
- Shaw LJ, Raggi P, Schisterman E, et al. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2003;228:826-33. [Crossref] [PubMed]
- Taylor AJ, Bindeman J, Feuerstein I, et al. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol 2005;46:807-14. [Crossref] [PubMed]
- Greenland P, LaBree L, Azen SP, et al. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 2004;291:210-5. [Crossref] [PubMed]
- Bybee KA, Lee J, Markiewicz R, et al. Diagnostic and clinical benefit of combined coronary calcium and perfusion assessment in patients undergoing PET/CT myocardial perfusion stress imaging. J Nucl Cardiol 2010;17:188-96. [Crossref] [PubMed]
- Fathala AL, Bukhari SQ, Shoukri M, et al. High prevalence of coronary artery calcification in Saudi patients with normal myocardial perfusion. Ann Saudi Med 2017;37:154-160. [Crossref] [PubMed]
- Kim YJ, Hur J, Lee HJ, et al. Meaning of zero coronary calcium score in symptomatic patients referred for coronary computed tomographic angiography. Eur Heart J Cardiovasc Imaging 2012;13:776-85. [Crossref] [PubMed]


