
The role of imaging in gastrointestinal bleed
Introduction
Gastrointestinal (GI) bleed accounts for approximately 20% of emergency visits and 2% of hospital admissions (1) and its incidence has been increasing (2,3). In a majority of patients, bleeding may stop spontaneously and intervention may not be required, but approximately 25% of patients develop massive or recurrent bleeding predisposing to increased morbidity and mortality, requiring intervention to identify the source and stop the hemorrhage (4). Identifying the source of the bleed can be challenging due to the wide range of potential causes, long length of the GI tract, and the intermittent nature of the bleed itself. Work-up of these patients is driven by the nature of the bleed and hemodynamic status of the patient. Work-up therefore involves a multidisciplinary approach including emergency physicians; internists; surgeons; gastroenterologists; diagnostic and interventional radiologists. Although many patients with GI bleeding can be identified and treated without imaging, radiology plays an important role in patients where endoscopy and/or medical management fails.
GI bleeding can be described in various ways depending upon the site or rate of bleeding. Based on anatomic location, it has traditionally been classified into two main categories: upper and lower GI bleed. Upper GI bleed (UGIB), refers to bleeding originating anywhere from the mouth to the ligament of Treitz, is the most common, accounting for approximately 75% of cases (5,6). Lower GI bleed (LGIB) refers to bleeding originating below the ligament of Treitz to the anus. The vast number of etiologies contributing to GI Bleeding have been described in Table 1. A second approach of classification takes into account the presentation as overt or “obscure/occult”. The latter was historically used to designate bleeding that could not be identified by either esophagogastroduodenoscopy (EGD) or colonoscopy. In 2015, the American College of Gastroenterology recommended that the term “obscure” GI bleeding be replaced by small bowel bleeding as the majority of cases of “obscure” GI bleeds are due to bleeding in the small bowel (7). The continued advancement of imaging tools, including video capsule endoscopy, deep enteroscopy, and radiologic imaging, has made this shift possible.

Full table
Role of imaging
Clinical differentiation of GI bleed as UGIB versus LGIB is the first step in diagnostic workup. Workup of overt UGIB starts with an upper endoscopy. Although upper endoscopy has a 92–98% sensitivity and a 3–100% specificity with the ability to treat patients effectively (1,2); various factors such as presence of a large amount of clot obscuring visibility of the site of bleeding or presence of co-morbidities and challenging anatomic locations can contribute to failure of upper endoscopic management. Workup of LGIB starts with colonoscopy. Colonoscopy however is not effective without adequate colon preparation which is a major limiting factor in the emergent setting. The diagnostic and therapeutic yield of colonoscopy in managing patients with LGIB also has wide variability in the range of 8–100% (4). Capsule endoscopy is widely popular in patients suspected of small bowel bleeds. However, the inability to use this modality in patients with presence of known small bowel strictures from any cause; the long duration of this test and a failure rate of 8.5% make radiologic imaging options also viable in the search for small bowel sites of bleed (5).
Imaging therefore plays a key role in all these patients with both overt and occult GI bleeding that are hemodynamically stable. Radiologic imaging modalities that are most frequently used in this setting include technetium 99m scintigraphy, computed tomography angiography (CTA), multiphase computed tomography enterography (CTE) and catheter angiography (CA). Each of these modalities has its own unique characteristic which we shall elaborate upon in this article.
The American college of Radiology has laid down appropriateness criteria for radiologic management of GI bleed (8).
According to the ACR appropriateness criteria for UGIB, upper endoscopy is the best initial modality and radiology does not play as significant a role as it does for LGIB, as an initial diagnostic modality. However, there are four situations/variants, according to ACR appropriateness criteria, where radiologic management is useful:
- Endoscopy reveals non-variceal arterial bleeding source: catheter angiography and CTA are almost equally useful. If the patient is hemodynamically unstable, catheter angiography is better. CTA may not be helpful if the bleed is intermittent;
- Endoscopy reveals non-variceal bleeding but does not identify a clear source: catheter angiography and CTA are equally useful;
- Endoscopy is negative: includes obscure UGIB. Catheter angiography, CTA, and multiphase CTE are comparable but can have false negatives. CTE has the highest radiation out of the three. Catheter angiography has the highest diagnostic yield but is a poor modality if variant arterial anatomy is present;
- Endoscopy is contraindicated: in these patients catheter angiography, CTA and CT abdomen with IV contrast are equally comparable.
According to the ACR appropriateness criteria for LGIB, there are four variant scenarios:
- Patients with active bleed and are hemodynamically stable: colonoscopy is the most appropriate initial modality of choice. In terms of radiological modalities, CTA and scintigraphy are equally useful but CTA has many added advantages to scintigraphy;
- Patients with active bleed and are hemodynamically unstable: catheter angiography is the most appropriate initial modality of choice;
- Rebleeding/ongoing bleeding post colonoscopic treatment for LGIB: catheter angiography is most appropriate;
- Intermittent or obscure bleed: multiphase CTE is comparable to capsule endoscopy and better than scintigraphy.
In this paper, we review the four main radiologic imaging tools, specifically, scintigraphy, CTA, multiphase CTE and CA. While acknowledging that variceal hemorrhage is an important cause of GI bleeding, we do not discuss it in detail in this review due to its complex physiology and management.
Scintigraphy
In 1979, Winzelberg et al. first described the use of technetium 99m (Tc-99m) labeled red blood cells (RBCs) to identify GI bleeds (9). Although additional methods of labeling the RBCs have been discovered and refined since that time, the initial concept has remained the same: after labeling the RBCs, intravascular injection is made followed by a dynamic acquisition, this allows for the visualization of extra-vascular deposition of the radiotracer into the bowel. Diagnostic criteria utilized when diagnosing a GI bleed with scintigraphy include: a focus of activity identified where none was seen initially, an increase in size over time, movement of tracer activity either retrograde or antegrade, and general conformity to the shape and location of the bowel (Figure 1).
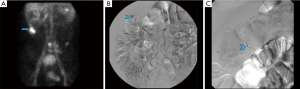
The benefits of this exam include the non-invasive nature, the lack of need for bowel preparation and the ability to identify both arterial and venous bleeds. It does not require iodinated contrast so it can be used without delay in patients with contrast allergies and those with limited renal function (10). Of the imaging methods, scintigraphy can identify the slowest active bleed: at a rate of approximately 0.05–0.10 mL/min (9-13). Finally, due to the stability of the radiolabel and physical half-life of Tc-99m, images can be acquired for up to 24 hours, which can be helpful in cases of intermittent bleeding (14).
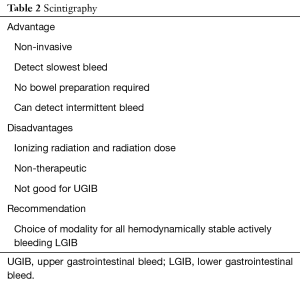
Unfortunately, scintigraphy has a few drawbacks that limit its use in most centers. The exam itself is time-consuming and may not be available in an emergency setting for acute bleeds (15). The exam, even when positive, does not provide any information as to the cause of the bleed (16). There are a few imaging pitfalls, which the interpreting radiologist should be aware of. Tagged RBCs can localize at sites distinct from bleeds, such as sites of splenosis, pancreatic pseudocysts and non-enteric bleeding (i.e., hematomas). Post-operative hyperemia, active Crohn disease, and hypervascular neoplasms can also appear as foci of uptake. These can give false positive results. A longstanding critique of scintigraphy was its inability to precisely identify the location of the bleed, thereby limiting its utility in guiding subsequent intervention. However, the addition of SPECT or SPECT-CT aids in localization (16-18) and should be considered when necessary. Table 2 summarizes the role of scintigraphy in GI Bleeds.
Full table
CTA
The most recent ACR appropriateness criteria guidelines for stable patients with lower GI bleeds give equal weight to Tc-99m RBC scan and CTA (8). Nevertheless, at most institutions, CTA has become the default first radiologic imaging option for most stable patients presenting with GI bleeding (19,20).
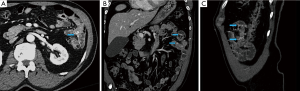
CTA is usually performed in three parts with an initial non-contrast phase, followed by arterial and portal venous phases without administration of oral contrast. The non-contrast phase is performed using a low-dose technique and its main utility is to identify pre-existing hyperdensities in the bowel such as a clot, pills, surgical clips or residual barium from the previous study, which may be confused for active bleeding. After reviewing the non-contrast images, the arterial phase images are carefully examined for a hyperattenuating focus that should increase in size on the subsequently acquired portal venous phase. The highest sensitivity is achieved by examining the arterial and portal venous phase images together (15). Slow or delayed bleeds may only be apparent on portal venous phase images. In some cases, while there is no evidence of active extravasation, a sentinel clot (seen as an unchanging hyperdensity) can be used to localize the site of recent bleeding. Even in situations in which no acute bleeding is identified, CTA can diagnose abnormalities that may be responsible for the bleeding, such as ischemia, inflammatory bowel disease, neoplasms (Figure 2) and arteriovenous malformations (AVMs) (16). CTA therefore provides an excellent road map guiding the next step in triaging patients to either endoscopic, surgical or angiographic management.
The advantages and disadvantages of CTA are elaborated in Table 3. CTA requires a significant amount of radiation, although efforts to reduce doses have become popular, including using very low dose for the non-contrast phase (19), eliminating the arterial or portal venous phase (21,22), and using dual-energy CT (23). CTA requires the rate of bleeding to be at least 0.3 mL/min, slightly higher than the rate of scintigraphy, but less than CA (24). Like scintigraphy, CTA requires active bleeding in order to see active extravasation, but a negative CTA may hold additional value beyond identifying structural lesions that likely account for the bleed. In 2015, Chan et al. demonstrated that patients with lower GI bleeding with negative CTAs were unlikely to bleed again, with only 22.6% requiring further radiologic or surgical intervention (25). All patients that did bleed again demonstrated hemodynamic instability, suggesting that this subset of patients with negative CTA can be managed conservatively with careful monitoring and further intervention should be considered when or if hemodynamic instability develops (25).

Full table
Multiphase CTE
Multiphase CTE is most useful in evaluating hemodynamically stable patients with intermittent and occult GI Bleeds, which commonly have an underlying small bowel source. The main difference between a multiphase CTE and routine CT or CTA is that in a CTE study, the small bowel lumen is distended with a bolus (about 1.5–2.0 litres) of a neutral oral contrast agent. This luminal distention allows optimal visualization of enhancement of the small bowel mucosa and wall following intravenous contrast administration, thereby increasing the sensitivity of detection of bleeding and non -bleeding abnormalities. At our institution we use very low (0.1%w/v) concentration of barium sulfate suspension (Volumen; Westbury, NY) that uses additives to make the fluid non-absorbable and palatable. Although exact multiphase CTE protocols vary among institutions, the general principles of intravenous contrast administration remain the same. These include imaging in the arterial phase (approximately 30 seconds) from the start of injection; followed by the enteric phase (approximately 50 seconds) and then the delayed phase (90–100 seconds) (Figure 3). For scanners with a dual-energy capability, scans can be acquired with lesser radiation and smaller volumes of intravenous contrast. Utilization of virtual unenhanced imaging capability on the dual energy scanners can also eliminate the need for multiphase scanning. The advantages and disadvantages of CTE are summarized in Table 4.
Full table
CA
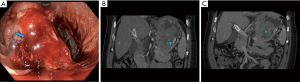
The use of selective arterial catheterization to identify GI bleeding was first described in the early 1960s (26). Since that time, the role of CA has increasingly evolved as endoscopic and non-invasive radiologic techniques have been developed and refined (27). Indeed, even though the ACR appropriateness criteria guidelines give the highest rating to CA in unstable lower GI bleeding patients, at our institution and many others, whenever possible, patients are routed to CTA prior to entering the angiography suite (8,26,27). The time spent to acquire the CTA pales in comparison to the time saved by the interventional radiologist who has a specific target for embolization and an understanding of any potential surgically altered or variant anatomy (28,29).
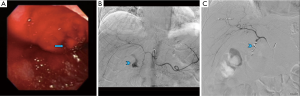
The chief advantage of CA is its ability to directly visualize and embolize a site of bleeding (30). Additionally, no bowel preparation is required (Figures 4,5). An important drawback is a requirement of a higher rate of bleeding for visualization. Historically, the minimum rate of bleeding required was 0.5–1 mL/min, though digital subtraction angiography may be able to identify slower bleeds (31). It is an invasive modality and thus has complications. Fortunately, major adverse events are rare, occurring in approximately 2–5% of cases (32,33). Non-targeted embolization and induction of bowel ischemia are two of the most feared complications, though the use of microcatheters and deploying the embolic agent as selectively as possible has decreased the incidence of these complications (34). Other complications include access site bleeding, hematoma or pseudoaneurysm and arterial dissection or spasm. Table 5 summarizes the utility of CA in GI bleeding.


Full table
Conclusions
GI bleeding is a common cause of presentation to the hospital and often requires the collaboration of a multi-specialty team, including gastroenterologists, surgeons and diagnostic and interventional radiologists. While many patients with GI bleed resolve spontaneously or can be treated with medical and endoscopic therapies, other cases require imaging tools beyond endoscopy. In this paper, we review the three radiologic imaging modalities in use today for GI bleeding: scintigraphy, CTA and catheter angiography.
Acknowledgments
Olga R. Brook, Associate Professor, Harvard Medical School; Clinical Director of CT, Abdominal Imaging and Interventional Radiology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Burke SJ, Golzarian J, Weldon D, et al. Nonvariceal upper gastrointestinal bleeding. Eur Radiol 2007;17:1714-26. [Crossref] [PubMed]
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015;149:1731-41.e3. [Crossref] [PubMed]
- Lanas A, Garcia-Rodriguez LA, Polo-Tomas M, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol 2009;104:1633-41. [Crossref] [PubMed]
- Hastings GS. Angiographic localization and transcatheter treatment of gastrointestinal bleeding. Radiographics 2000;20:1160-8. [Crossref] [PubMed]
- Rondonotti E, Herrerias JM, Pennazio M, et al. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc 2005;62:712-6. [Crossref] [PubMed]
- Wortman JR, Landman W, Fulwadhva UP, et al. CT angiography for acute gastrointestinal bleeding: what the radiologist needs to know. Br J Radiol 2017;90:20170076. [Crossref] [PubMed]
- Gerson LB, Fidler JL, Cave DR, et al. ACG clinical guideline: Diagnosis and management of small bowel bleeding. Am J Gastroenterol 2015;110:1265-87. [Crossref] [PubMed]
- ACR-Appropriateness-CriteriaACR Appropriateness Criteria for LGIB. 2014. Available online: https://www.acr.org/Clinical-Resources/
- Winzelberg GG, McKusick KA, Strauss HW, et al. Evaluation of gastrointestinal bleeding by red blood cells labeled in vivo with technetium-99m. J Nucl Med 1979;20:1080-6. [PubMed]
- Grady E. Gastrointestinal bleeding scintigraphy in the early 21st century. J Nucl Med 2016;57:252-9. [Crossref] [PubMed]
- Zuckier LS. Acute gastrointestinal bleeding. Semin Nucl Med 2003;33:297-311. [Crossref] [PubMed]
- Howarth DM. The role of nuclear medicine in the detection of acute gastrointestinal bleeding. Semin Nucl Med 2006;36:133-46. [Crossref] [PubMed]
- Ponzo F, Zhuang H, Liu FM, et al. Tc-99m sulfur colloid and Tc-99m tagged red blood cell methods are comparable for detecting lower gastrointestinal bleeding in clinical practice. Clin Nucl Med 2002;27:405-9. [Crossref] [PubMed]
- Ziessman H, O’Malley J, Thrall J. Nuclear medicine: The requisites. 4th ed. Philadelphia, PA: Elsevier Saunders, 2013:307-21.
- Soto JA, Park SH, Fletcher JG, et al. Gastrointestinal hemorrhage: evaluation with MDCT. Abdom Imaging 2015;40:993-1009. [Crossref] [PubMed]
- Raman SP, Fishman EK. Computed tomography angiography of the small bowel and mesentery. Radiol Clin N Am 2016;54:87-100. [Crossref] [PubMed]
- Bentley BS, Tulchinsky M. SPECT/CT helps in localization and guiding management of small bowel gastrointestinal hemorrhage. Clin Nucl Med 2014;39:94-6. [Crossref] [PubMed]
- Dolezal J, Vizda J, Kopacova M. Single-photon emission computed tomography enhanced Tc-99m-pertechnetate disodium-labeled red blood cell scintigraphy in the localization of small intestine bleeding: a single-centre twelve-year study. Digestion 2011;84:207-11. [Crossref] [PubMed]
- Kim BS, Li BT, Engel A, et al. Diagnosis of gastrointestinal bleeding: A practical guide for clinicians. World J Gastrointest Pathophysiol 2014;5:467-78. [Crossref] [PubMed]
- Wells ML, Hansel SL, Bruining DH, et al. CT for evaluation of acute GI Bleeding. Radiographics 2018;38:1089-107. [Crossref] [PubMed]
- Artigas JM, Marti M, Soto JA, et al. Multidetector CT angiography for acute gastrointestinal bleeding: technique and findings. Radiographics 2013;33:1453-70. [Crossref] [PubMed]
- Kim JW, Shin SS, Yoon W, et al. Diagnosis of acute gastrointestinal bleeding: comparison of the arterial, the portal, and the combined set using 64-section computed tomography. J Comput Assist Tomogr 2011;35:206-11. [Crossref] [PubMed]
- Sun H, Xue HD, Wang YN, et al. Dual-source dual-energy computed tomography angiography for active gastrointestinal bleeding: a preliminary study. Clin Radiol 2013;68:139-47. [Crossref] [PubMed]
- Kuhle WG, Sheiman RG. Detection of active colonic hemorrhage with use of helical CT: findings in a swine model. Radiology 2003;228:743-52. [Crossref] [PubMed]
- Chan V, Tse D, Dixon S, et al. Outcome following a negative CT angiogram for gastrointestinal hemorrhage. Cardiovasc Intervent Radiol 2015;38:329-35. [Crossref] [PubMed]
- Nusbaum M, Baum S. Radiographic demonstration of unknown sites of gastrointestinal bleeding. Surg Forum 1963;14:374-5. [PubMed]
- Zurkiya O, Walker TG. Angiographic evaluation and management of nonvariceal gastrointestinal hemorrhage. AJR 2015;205:753-63. [Crossref] [PubMed]
- Speir EJ, Ermentrout RM, Martin JG. Management of acute lower gastrointestinal bleeding. Tech Vasc Interv Radiol 2017;20:258-62. [Crossref] [PubMed]
- Storace M, Martin JG, Shah J, et al. CTA as an adjuvant tool for acute intra-abdominal or gastrointestinal bleeding. Tech Vasc Interv Radiol 2017;20:248-57. [Crossref] [PubMed]
- Jacovides CL, Nadolski G, Allen SR, et al. Arteriography for lower gastrointestinal hemorrhage: Role of preceding abdominal computed tomographic angiogram in diagnosis and localization. JAMA Surg 2015;150:650-6. [Crossref] [PubMed]
- Krüger K, Heindel W, Dölken W, et al. Angiographic detection of gastrointestinal bleeding. An experimental comparison of conventional screen-film angiography and digital subtraction angiography. Invest Radiol 1996;31:451-7. [Crossref] [PubMed]
- Walker TG, Salazar GM, Waltman AC. Angiographic evaluation and management of acute gastrointestinal hemorrhage. World J Gastroenterol 2012;18:1191-201. [Crossref] [PubMed]
- Tan KK, Wong D, Sim R, et al. Superselective embolization for lower gastrointestinal hemorrhage: an institutional review over 7 years. World J Surg 2008;32:2707-15. [Crossref] [PubMed]
- Funaki B, Kostelic JK, Lorenz J, et al. Superselective microcoil embolization of colonic hemorrhage. AJR Am J Roentgenol 2001;177:829-36. [Crossref] [PubMed]


