
Prevalence and impact of non-cardiovascular comorbidities among older adults hospitalized for non-ST segment elevation acute coronary syndrome
Introduction
Coronary artery disease (CAD) is a leading cause of global morbidity and mortality (1,2). Its acute (and most ominous) manifestation, acute coronary syndrome (ACS), is associated with >2.5 million hospitalizations annually worldwide (3). Unstable angina (UA) and non-ST segment elevation myocardial infarction (NSTEMI) share similar clinical presentations, but differ in severity and are collectively referred to as non-ST elevation ACS (NSTE-ACS). Together, they account for over 60% of ACS cases (4,5).
The risk of ACS increases with age and the co-existence of risk factors such as hypertension and smoking (4,6,7). In Australia, the recorded cases of ACS increased by 79% from 1993 to 2008 for acute myocardial infarction (AMI) and 33% for UA, resulting in over 90,000 hospitalizations in 2008 (8). More than 8,000 Australians died from AMI in 2016, among whom over 80% were aged 65 years and over (9). In 2009, the direct healthcare costs associated with ACS in Australia exceeded AUD $1.8 billion (10).
ACS clinical guidelines are largely based on trials in which the elderly and people with comorbidities were under-represented (11,12). However, the majority of ACS patients seen in routine clinical practice are likely to belong to this group. In Sweden, around half of cardiologists reported treating elderly NSTE-ACS patients with multimorbidity daily (13). Approximately 60% of patients with ACS in England and Wales were found to have multimorbidity (14). One in every 3 patients hospitalized for ACS in Switzerland had two or more other cardiovascular conditions (15). In Australia, similar patterns are observed (16,17).
To optimize the clinical management of patients with ACS, better knowledge of the impact of comorbidities is vital (18,19). Prior studies that have utilized comorbidity indices such as the Charlson comorbidity index (CCI) and the CAD-specific comorbidity index have associated higher global comorbidity with poorer outcomes among patients with ACS (20,21). However, the extent of, and the impact of NCCs on outcomes experienced by ACS patients has not been well studied. Defining the burden of and the effect of NCCs among ACS patients might help to raise awareness about the clinical relevance of NCCs among clinicians which could improve the assessment of patients’ vulnerability, and also inform decision making about the care delivered to such patients.
In this study, we sought to characterize the patterns and impact of NCCs on the length of stay (LOS) and mortality among older adults hospitalized for NSTE-ACS.
Methods
Study design and patient selection
This was a retrospective study which utilized administrative data from the Alfred Hospital, a major tertiary hospital in Melbourne, Australia. We assembled a cohort of all consecutive adults aged 65 years and over, who were hospitalized for NSTE-ACS between July 2013 and December 2015. NSTE-ACS was defined by the International Classification of Diseases, Version 10, Australian Modification (ICD-10-AM) codes (22). The relevant NSTE-ACS codes were I21.4 (NSTEMI) and I21.0 (UA). Patients were eligible for inclusion if at least one of the above codes was listed as a primary reason (diagnosis) for their admission.
Non-cardiovascular comorbidities (NCCs)
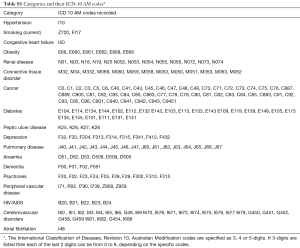
The CCI has been widely used in clinical research to address the confounding effect of comorbidities, predict outcomes, as well as to standardize comorbidities abstracted from medical records or administrative databases (20,23,24). The CCI incorporates 17 comorbidities that were originally evaluated for their prognostic impact on 1-year mortality risk (24). To determine a set of relevant NCCs to examine in our study, we selected a priori those NCCs incorporated in the CCI—dementia, chronic pulmonary disease (CPD), connective tissue disorders (CTD), peptic ulcer disease, diabetes mellitus, liver disease, malignancy, HIV and renal disease. This approach to selecting NCCs has previously being adopted by other studies that have examined the impact of NCCs on hospitalisation outcomes (25). In addition, while not included in the CCI, we added anemia, depression and psychoses because prior studies have identified these as significant prognostic factors in ACS patients (26,27). Furthermore, we included obesity given its growing public health burden (28). Table S1 provides the relevant codes used for ascertaining various comorbidities.
Full table
Outcomes
We examined LOS and in-hospital mortality among the study cohort. In-hospital fatality rate was calculated as the ratio of the number of cases ending in death (from any cause) to the total number of cases. LOS was calculated as the period from the time of admission to the time of separation (death or discharged alive).
Covariates
For each patient, we collected demographic data on date of birth, sex, place of birth, postcode of residence, marital status and whether or not the patient identified as an Aboriginal and/or Torres Strait Islander. Postcode of residence was used to describe socioeconomic status as per the Australian Bureau of Statistics’ Socio-Economic Indices for Areas (SEIFA) and the Index of Relative Socio-Economic Advantage and Disadvantage (IRSAD) (29). The IRSAD is a general socioeconomic index that summarizes a range of information about the economic and social conditions of people and households within an area. Clinical data included the type of NSTE-ACS, information on coronary interventional procedures [e.g., coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI)], admission to intensive care unit (ICU), and presence or absence of any complications during hospital stay. Information regarding smoking, hypertension and atrial fibrillation (AF), and other comorbidities that are captured in the CCI were also collected.
Statistical analyses
Descriptive statistics were used to summarize baseline characteristics. Variables were compared across patients with differing levels of NCCs using the χ2 test for proportions, one-way analysis of variance (ANOVA) for means and Kruskal-Wallis rank test for the comparison of medians. The prevalence of each of the 13 NCCs was calculated as the ratio of the patients with that comorbidity to the overall number of NSTE-ACS patients. The occurrence of NCC dyads was examined by clustering the individual conditions per patient and estimating the prevalence for each combination with a focus on the five most prevalent dyads. The expected (E) prevalence of each dyad was calculated by multiplying the total prevalence of the single NCC within the dyad by each other (e.g., for NCC dyad XY, the expected prevalence would be prevalence of comorbid X × prevalence of comorbid Y) (17,30). The observed (O) and expected (E) prevalence of each NCC dyad were compared via a two-sample test of proportion (31).
We performed three different analyses. In the first, individual NCCs were considered as exposures. In the second analysis, the exposure was considered as the cumulative number of NCCs and the patients were classified into three groups based on having 0, 1 and ≥2 NCCs. In the third set of analyses, the occurrence of NCC dyads was considered as the exposure whereby for each dyad, patients were grouped into whether they had none, only one or both of the component NCCs. For example, for dyad XY, patients would be grouped into: (I) with neither X nor Y; (II) with X but not Y; (III) with Y but not X; (IV) with both X and Y.
The associations between NCCs and LOS and in-hospital mortality were examined by means of a negative binomial regression (NBR) model and Cox proportional hazards regression model, respectively. Backward stepwise approach was applied to select covariates (from the possible list in Table 1) in multivariable NBR and Cox models with removal set at probability of 0.20 (25). The exposure of interests was forced into the respective models. Assessments of model fit, collinearity and interactions were performed. Survival patterns were depicted by Kaplan-Meier curves. The log-rank test was used to compare the survival between ties. Unless otherwise specified, a priori statistical significance was set at P<0.05. All analyses were performed with STATA (version 15/1C, Stata Corp, College Station, Texas, USA).
Full table
Ethics approval
The study received approval from the Alfred Hospital Human Research Ethics Committee (project number 146/17).
Results
Cohort characteristics
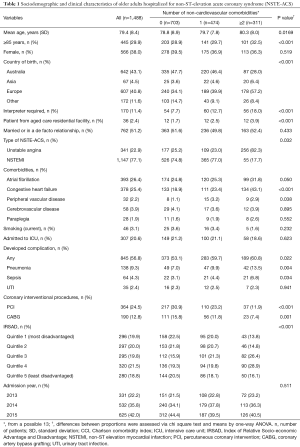
A total of 1,488 older adults were included in the analysis, of whom 77.1% and 22.9% were hospitalized for NSTEMI and UA, respectively. The mean age of the cohort was 79.4 (SD 8.4) years and 62.0% were males. Approximately 2.4% were patients from an aged care residential facility. Over half (56.9%) of the patients were born overseas and 11.4% were non-English speaking and required an interpreter. A total of 26.4%, 60.9%, 25.4%, 3.9%, 2.2% and 3.1% patients had AF, hypertension, chronic heart failure, cerebrovascular disease, peripheral vascular disease and currently smoked, respectively. Nearly 1 in 8 (12.8%) had a previous history of CABG and a quarter (24.5%) with PCI. One in five (20.6%) patients were admitted to the ICU. Overall, 31.9% had only one NCC, whereas 20.9% had two or more. Table 1 summarizes the sociodemographic and clinical characteristics of the NSTE-ACS according to their number of NCCs. No gender differences among the various groups of patients were observed, but patients without NCCs were younger than those with NCCs. Patients with NCCs were more likely to be born overseas than in Australia. The occurrence of AF, chronic heart failure and peripheral vascular disease were less frequent among patients without NCCs.
Prevalence of NCCs
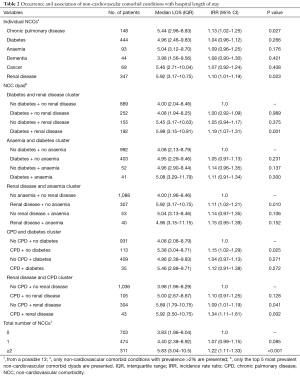
The prevalence of diabetes, renal disease, CPD, anemia, cancer and dementia among the NSTE-ACS patients were 29.8%, 23.3%, 10.0%, 6.3%, 4.6% and 3.0%, respectively. The prevalence of the remaining NCCs were each less than 2%. We observed 38 unique NCC dyads. The five most prevalent NCC dyads were diabetes + renal disease (12.9%), CPD + renal disease (2.9%), diabetes + anemia (2.8%), anemia + renal disease (2.7%) and diabetes + CPD (2.4%). The expected prevalence of the top five NCC dyads were diabetes + renal disease (6.9%), CPD + renal disease (2.3%), diabetes + anemia (1.9%), anemia + renal disease (1.5%) and diabetes + pulmonary disease (2.9%). The differences in expected (E) and observed (O) prevalence of NCC dyads were significant only for diabetes + renal disease and anemia + renal disease (i.e., O/E>1, P value <0.05). For the remaining top 5 NCC dyads, no differences in the expected and observed prevalence were observed.
LOS
The median LOS of the cohort was 4.31 days [interquartile range (IQR) 2.17 to 8.92 days]. The unadjusted median LOS among patients with UA was 2.88 days (IQR 1.13–6.92) compared to 5.04 days (IQR 2.67–9.68) for patients with NSTEMI (P value for difference <0.001). The unadjusted median LOS for individual NCCs and their groupings are presented in Table 2. Having renal disease [incidence rate ratio (IRR) 1.10; 95% CI: 1.01–1.19; P=0.023] or CPD (IRR 1.13; 95% CI: 1.02–1.25; P=0.027) was associated with increased likelihood of longer LOS than not having these comorbidities. The unadjusted median LOS among patients with 0, 1 and ≥2 NCCs were 3.83 days (IQR 1.96–8.04), 4.40 days (IQR 2.38–8.92) and 5.83 days (IQR 3.04–10.5) (P value for difference <0.001), respectively. Compared to being without NCCs, having one NCC was not associated with greater likelihood of increased LOS (IRR 1.07; 95% CI: 0.99–1.15; P=0.085). However, having two or more NCCs was associated with a 22% increased likelihood of longer LOS compared to not having any NCCs (IRR 1.22; 95% CI: 1.11–1.33; P<0.001). Patients with diabetes but no renal disease (IRR 1.00; 95% CI: 0.92–1.09; P=0.989) and patients with renal disease but no diabetes (IRR 1.05; 95% CI: 0.94–1.17; P=0.375) were no more likely to experience a longer LOS than patients without diabetes or renal disease. Conversely, patients with both diabetes and renal disease experienced a 19% (IRR 1.19; 95% CI: 1.07–1.31; P=0.001) greater likelihood of increased LOS compared to patients without diabetes or renal disease. Similarly, in comparison to patients with neither CPD nor renal disease, those with CPD but no renal disease did not experience a significant increase in the likelihood of longer LOS (IRR 1.10; 95% CI: 0.97–1.25; P=0.128). On the other hand, the co-existence of CPD and renal disease was associated with a 34% increased likelihood of longer LOS (IRR 1.34; 95% CI: 1.11–1.61; P=0.002).
Full table
In-hospital mortality
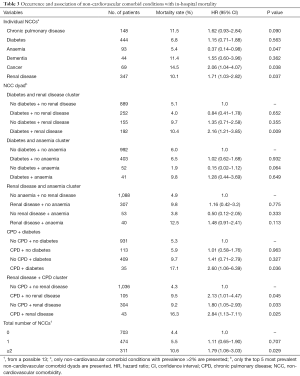
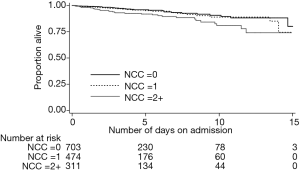
Overall, in-hospital death occurred in 6.1% of the cohort, 2.6% in patients with UA and 7.1% among patients with NSTEMI (P value for difference =0.003). The unadjusted mortality rates for individual NCCs and their groupings are presented in Table 3. Having cancer was associated with over two times greater likelihood of in-hospital death compared to not having cancer [hazard ratio (HR), 2.06; 95% CI: 1.04–4.07; P=0.038]. Similarly, NSTE-ACS patients with renal disease were about 71% more likely to die compared to those without renal disease (HR, 1.71; 95% CI: 1.03–2.82; P=0.037). The unadjusted in-hospital mortality rate among patients with 0, 1 and ≥2 NCCs were 4.4%, 5.5% and 10.6%, respectively (P value for difference =0.001). Figure 1 depicts the Kaplan-Meier survival curves of the NSTE-ACS patients according to their total number of NCCs. The presence of single NCC was not associated with a significant increase in the likelihood of in-hospital death (HR, 1.11; 95% CI: 0.65–1.90; P=0.707), whereas having two or more was associated with 79% increased likelihood of in-hospital death (HR, 1.79; 95% CI: 1.06–3.03; P=0.029), compared to not having any NCC. Compared to patients without diabetes or renal disease, those with both diabetes and renal disease were more than twice as likely to die in hospital (HR 2.16, 1.21–3.85; P=0.009). Patients with CPD but no diabetes were no more likely to die than patients with neither (HR, 1.01; 95% CI: 0.58–1.76; P=0.963). However, their co-existence was associated with an increased risk of death (HR, 2.60; 95% CI: 1.06–6.39; P=0.036).
Full table
Discussion
In this large short-term study, there was a high burden of NCCs among older adults hospitalized for NSTE-ACS. Some NCC dyads also occurred more frequently than would have been expected. Overall, patients with a higher burden of NCCs experienced a longer LOS and higher mortality rates during hospitalization. In addition, certain NCC dyads exhibited multiplicative effects such that their impact on patients’ LOS or likelihood of in-hospital death exceeded the sum of the individual contributory effects of the component conditions.
In this study population, more than half of the patients had at least one NCC and around one-fifth had two or more. Previous international studies have reported varying prevalence of NCCs among patients with ACS, ranging from 10% among AMI patients in Vietnam (32) to over 60% among AMI patients in the United States (33,34). Regardless, the majority of prior studies have focused on populations which were younger than our study participants, with some also including a mix of STEMI and NSTEMI cases. Among the 13 NCCs examined, diabetes was the most prevalent. Our results are in accord with the findings from other contemporary studies. In the Worcester Heart Attack Study that examined trends in the frequency of multiple comorbidities among patients hospitalised for AMI from 1990 to 2007, diabetes was the most prevalent NCC (33). Taken together with prior research, our results suggest a high burden of NCCs among ACS patients.
Although the occurrence of NCC dyads was expected, the observed prevalence of some dyads exceeded the expected prevalence, suggestive of possible clustering. There are multiple explanations for such a high rate of NCC dyads in our study population. First, due to the link between ageing and the development of chronic diseases, the occurrence of multiple pathologies is prevalent among older adults. A recent systematic review estimated that more than 66% of older adults in ambulatory settings had two or more chronic medical conditions (35). Second, in light of the strong pathophysiological associations between diseases such as diabetes and renal disease, “causal comorbidity” has likely contributed to clustering of these conditions in our elderly patient population. In a recent Mendelian randomisation analysis involving 11,502 participants, the study authors concluded there was evidence for a causal association between type 2 diabetes and chronic kidney disease (36). Moreover, due to reduced production of erythropoietin as a consequence of kidney damage, anemia is often common among patients with renal disease (37), which may explain the higher co-occurrence of renal disease and anemia among our study population.
We found that the occurrence of an additional NCC on top of an existing one may sometimes result in a multiplicative rather than an expected additive effect. For example, while patients with renal disease but no CPD were slightly (9%) more likely to experience longer LOS than those with neither condition, their co-existence was associated with a 34% increased risk of longer LOS. Similarly, whiles patients with diabetes but no renal disease did not experience significantly higher risk of deaths than those with neither condition, their co-existence was associated with over two times increased risk of in-hospital death. The extent to which the addition of another NCC to an existing one increases the biological vulnerability of ACS patients deserves further exploration.
Our observation that the occurrence of multiple NCCs is associated with poor short-term survival and increased hospital LOS may also reflect sub-optimal care for patients with comorbidities, given that the majority of clinical trials and, in turn, practice guidelines, are almost exclusively single-disease focused (11,12). In practice, patients with comorbidities are vulnerable to higher rates of complications from ACS treatment, such as bleeding, owing to factors such as drug–drug interactions and drug-disease interactions (33,38). This may result in complex clinical scenarios for which standard clinical management guidelines may be irrelevant. Thus, our results suggest that further population-based data on the use and impact of contemporary ACS treatments and guidelines in patients with multiple comorbid conditions are needed. Importantly, policy makers or authoritative professional bodies who are responsible for monitoring clinical protocols and for developing ACS guidelines need to acknowledge the existence of significant numbers of patients who are affected by multiple comorbidities. Although, recent European Society of Cardiology (ESC) guidelines for the management of ACS in patients presenting without persistent ST-segment elevation has highlighted the need for greater considerations of comorbidities (39), there remains much room for improvement in the recognition of comorbidities in ACS guidelines. Furthermore, clinicians whose specialty focuses on specific conditions also need to be aware of the high proportion of their patients with multiple chronic conditions in order to know how to coordinate their care with other clinical providers.
A number of limitations to our study warrant mention. First, our analysis was based on hospital administrative hospital data that were not collected for research purposes. Some studies have found that administrative data may lack completeness and at times under-estimate comorbidities (40). Nonetheless, the quality of administrative data has improved significantly in recent times, resulting in their increased use for research purposes (41). Moreover, a recent study found good agreement between medical records and claims (administrative) data when evaluating the presence of comorbidities such as HIV, renal disease, dementia, cancers, cerebrovascular disease and liver disease (42). Furthermore, despite the limitations of administrative data, they outperform snapshot chart reviews for comorbidity assessment (43). Methodologically, we also utilized all available patient records (outpatient attendance, emergency services and all hospitalisations records) prior to admission for the ACS which increased the likelihood of any comorbidities being documented/identified. However, information was not available in the administrative dataset for important covariates such as disease severity, laboratory and imaging results and drug treatments (44). Second, some of our sub-group analyses may have been underpowered and a larger-sample based study may be needed to further explore the direction of impact. Third, our sample population was selected from a single hospital, therefore, selection bias may limit the generalisability of our findings. Fourth, not all NCCs have been examined in this study and as such future studies could examine the prognostic impact of other NCCs not examined in this study. Lastly, the use of only in-hospital data meant that we could not examine the impact of NCCs on longer-term post-discharge outcomes among the ACS patients.
Conclusions
Among older patients hospitalized with NSTE-ACS, there is a high burden of NCCs. Some NCC dyads also occurred more frequently than expected suggestive of a possible clustering. In general, a higher burden of NCCs correlated with increased LOS and poorer survival. These findings highlight the need for contemporary ACS clinical treatment guidelines to recognise and incorporate protocols for the treatment of individuals with multiple chronic conditions to reduce the occurrence of adverse outcomes.
Acknowledgments
R Ofori-Asenso is supported by a Monash Graduate Scholarship and Monash International Postgraduate Research Scholarship for his doctoral studies. S Zoungas is funded by a National Health and Medical Research Council Senior Research Fellowship.
Footnote
Conflicts of Interest: S Zoungas reports past participation in advisory boards/educational meetings/research on behalf of Monash University (for work unrelated to this paper) with AstraZeneca Pty Ltd., Eli Lilly Australia Pty Ltd, Merck Sharp & Dohme (Australia) Pty Ltd and Novo Nordisk Pty Ltd. D Liew reports past participation in advisory boards and/or receiving honoraria from Abbvie, Astellas, AstraZeneca, Bristol-Myers Squibb, Novartis, Pfizer, Sanofi and Shire for work unrelated to this study. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Alfred Hospital Human Research Ethics Committee (project Number 146/17).
References
- Roth GA, Johnson C, Abajobir A, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1-25. [Crossref] [PubMed]
- Mendis S. Global progress in prevention of cardiovascular disease. Cardiovasc Diagn Ther 2017;7:S32-8. [Crossref] [PubMed]
- Grech ED, Ramsdale DR. Acute coronary syndrome: unstable angina and non-ST segment elevation myocardial infarction. BMJ 2003;326:1259-61. [Crossref] [PubMed]
- Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc 2009;84:917-38. [Crossref] [PubMed]
- Dai X, Busby-Whitehead J, Alexander KP. Acute coronary syndrome in the older adults. J Geriatr Cardiol 2016;13:101-8. [PubMed]
- North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res 2012;110:1097-108. [Crossref] [PubMed]
- Rajpurohit N, Ayaz SZ, Yee J, et al. Review of acute coronary syndromes: diagnosis and management of unstable angina and non ST-elevation myocardial infarction. South Dakota Med 2015;68:71-3, 75.
- Hyun KK, Essue BM, Woodward M, et al. The household economic burden for acute coronary syndrome survivors in Australia. BMC Health Serv Res 2016;16:636. [Crossref] [PubMed]
- Australian Bureau of Statistics. Causes of Death 2016, ABS cat. no. 3303.0, September. 2017 [cited July 07 2018]. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/3303.0~2016~Main%20Features~Australia's%20leading%20causes%20of%20death,%202016~3
- Access Economics. The economic costs of heart attack and chest pain (Acute Coronary Syndrome). 2009 [cited July 27 2018]. Available online: (emilable.pdfhttps://www.baker.edu.au/Assets/Files/FullReport%20-%20the%20economic%20costs%20of%20heart%20attack%20and%20chest%20pain%20
- Rich MW, Chyun DA, Skolnick AH, et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population: A Scientific Statement From the American Heart Association, American College of Cardiology, and American Geriatrics Society. J Am Coll Cardiol 2016;67:2419-40. [Crossref] [PubMed]
- Dhruva SS, Redberg RF. Variations between clinical trial participants and Medicare beneficiaries in evidence used for Medicare national coverage decisions. Arch Intern Med 2008;168:136-40. [Crossref] [PubMed]
- Ekerstad N, Lofmark R, Carlsson P. Elderly people with multi-morbidity and acute coronary syndrome: doctors' views on decision-making. Scand J Public Health 2010;38:325-31. [Crossref] [PubMed]
- Hall M, Dondo TB, Yan AT, et al. Multimorbidity and survival for patients with acute myocardial infarction in England and Wales: Latent class analysis of a nationwide population-based cohort. PLoS Med 2018;15:e1002501. [Crossref] [PubMed]
- Canivell S, Muller O, Gencer B, et al. Prognosis of cardiovascular and non-cardiovascular multimorbidity after acute coronary syndrome. PloS One 2018;13:e0195174. [Crossref] [PubMed]
- Caughey GE, Vitry AI, Gilbert AL, et al. Prevalence of comorbidity of chronic diseases in Australia. Bmc Public Health 2008;8:221. [Crossref] [PubMed]
- Ofori-Asenso R, Ilomaki J, Curtis AJ, et al. Patterns of Medication Dispensation for Multiple Comorbidities among Older Adults in Australia. Pharmacy 2018. [Crossref] [PubMed]
- Boyd CM, Vollenweider D, Puhan MA. Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. PLoS One 2012;7:e41601. [Crossref] [PubMed]
- Schmidt M, Jacobsen JB, Lash TL, et al. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ 2012;344:e356. [Crossref] [PubMed]
- Rashid M, Kwok CS, Gale CP, et al. Impact of co-morbid burden on mortality in patients with coronary heart disease, heart failure, and cerebrovascular accident: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes 2017;3:20-36. [Crossref] [PubMed]
- Radovanovic D, Seifert B, Urban P, et al. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002-2012. Heart 2014;100:288-94. [Crossref] [PubMed]
- Independent Hospital Pricing Authority. Emergency Department ICD-10-AM (Tenth Edition) Principal Diagnosis Short List. 2017 [cited July 29 2018]. Available online: https://www.ihpa.gov.au/sites/g/files/net636/f/Documents/ed_icd-10-am_principal_diagnosis_short_list_user_guide_tenth_edition.pdf
- Roffman CE, Buchanan J, Allison GT. Charlson Comorbidities Index. J Physiother 2016;62:171. [Crossref] [PubMed]
- Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245-51. [Crossref] [PubMed]
- Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998-1005. [Crossref] [PubMed]
- Thune JJ, Signorovitch J, Kober L, et al. Effect of antecedent hypertension and follow-up blood pressure on outcomes after high-risk myocardial infarction. Hypertension 2008;51:48-54. [Crossref] [PubMed]
- Lawler PR, Filion KB, Dourian T, et al. Anemia and mortality in acute coronary syndromes: a systematic review and meta-analysis. Am Heart J 2013;165:143-53.e5. [Crossref] [PubMed]
- Houston DK, Nicklas BJ, Zizza CA. Weighty concerns: the growing prevalence of obesity among older adults. J Am Diet Assoc 2009;109:1886-95. [Crossref] [PubMed]
- Australian Bureau of Statistics. Socio-Economic Indexes for Areas. 2018 [cited May 15 2018]. Available online: http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa
- van Oostrom SH, Picavet HS, van Gelder BM, et al. Multimorbidity and comorbidity in the Dutch population - data from general practices. BMC Public Health 2012;12:715. [Crossref] [PubMed]
- Loza E, Jover JA, Rodriguez-Rodriguez L, et al. Observed and expected frequency of comorbid chronic diseases in rheumatic patients. Ann Rheum Dis 2008;67:418-21. [Crossref] [PubMed]
- Nguyen HL, Nguyen QN, Ha DA, et al. Prevalence of Comorbidities and Their Impact on Hospital Management and Short-Term Outcomes in Vietnamese Patients Hospitalized with a First Acute Myocardial Infarction. Plos One 2014;9:e108998. [Crossref] [PubMed]
- McManus DD, Nguyen HL, Saczynski JS, et al. Multiple cardiovascular comorbidities and acute myocardial infarction: Temporal trends (1990-2007) and impact on death rates at 30 days and 1 year. Clin Epidemiol 2012;4:115-23. [PubMed]
- Chen HY, Saczynski JS, McManus DD, et al. The impact of cardiac and noncardiac comorbidities on the short-term outcomes of patients hospitalized with acute myocardial infarction: A population-based perspective. Clin Epidemiol 2013;5:439-48. [PubMed]
- Ofori-Asenso R, Chin KL, Curtis AJ, et al. Recent Patterns of Multimorbidity Among Older Adults in High-Income Countries. Popul Health Manag 2019;22:127-37. [Crossref] [PubMed]
- Xu M, Bi Y, Huang Y, et al. Type 2 Diabetes, Diabetes Genetic Score and Risk of Decreased Renal Function and Albuminuria: A Mendelian Randomization Study. EBioMedicine 2016;6:162-70. [Crossref] [PubMed]
- Lamb EJ, Levey AS, Stevens PE. The Kidney Disease Improving Global Outcomes (KDIGO) guideline update for chronic kidney disease: evolution not revolution. Clin Chem 2013;59:462-5. [Crossref] [PubMed]
- Doos L, Roberts EO, Corp N, et al. Multi-drug therapy in chronic condition multimorbidity: a systematic review. Fam Pract 2014;31:654-63. [Crossref] [PubMed]
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. [Crossref] [PubMed]
- Leal JR, Laupland KB. Validity of ascertainment of co-morbid illness using administrative databases: a systematic review. Clin Microbiol Infect 2010;16:715-21. [Crossref] [PubMed]
- Freitas A, Lema I, da Costa-Pereira A. Comorbidity coding trends in hospital administrative databases. Adv Intell Syst 2016;445:609-17. [Crossref]
- Seo HJ, Yoon SJ, Lee SI, et al. A comparison of the Charlson comorbidity index derived from medical records and claims data from patients undergoing lung cancer surgery in Korea: a population-based investigation. BMC Health Serv Res 2010;10:236. [Crossref] [PubMed]
- Luthi JC, Troillet N, Eisenring MC, et al. Administrative data outperformed single-day chart review for comorbidity measure. Int J Qual Health Care 2007;19:225-31. [Crossref] [PubMed]
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676-82. [Crossref] [PubMed]


