A network meta-analysis for safety of endothelin receptor antagonists in pulmonary arterial hypertension
Introduction
Pulmonary arterial hypertension (PAH) is a kind of life-threatening disease characterized by progressively incremental pulmonary vascular resistance and pulmonary artery pressure, ultimately leading to right heart failure and death (1). Drugs for PAH-specific therapy, which target endothelial dysfunction or other specific pathways, have been approved by US Food and Drug Administration (FDA) (1). Currently, five classes of agents were applied for the treatment of PAH, which include endothelin receptor antagonists (ERAs), phosphodiesterase type 5 inhibitors, prostanoids, selective prostacyclin receptor agonists and soluble guanylate cyclase stimulators (1). Regarding ERAs, until now, four ERAs (bosentan, ambrisentan, macitentan and sitaxsentan), which exert antiproliferative effects and vasodilator by binding to endothelin receptor type A (ETA) and/or type B (ETB) in pulmonary vascular smooth muscle cells, have been demonstrated to significantly improve hemodynamics, exercise symptoms, capacity, and to slow clinical worsening in trials (2-5). Whereas, along with the widespread clinical use, ERAs safety was gradually reported (6-8). Sitaxsentan, as the first selective ERA antagonist, was withdrawn from the market worldwide owing to some reports of fatal liver injury in patients with PAH (9,10). Peripheral edema, abnormal liver function and anemia have been reported as the main adverse effects of ERAs in previous study. Whereas, most of the studies involved relatively small samples, and each study has issued a small number of adverse drug events. In addition, no head-to-head comparisons has been addressed to assess the ERAs safety in PAH patients. To boost precision results for decision making, it is necessary to evaluate current safety evidence of ERAs in patients with PAH by combining the results of individual studies on the basis of direct- and indirect comparison, as well as to rank ERAs in evidence network.
Methods
Data sources and searches
The present study of systematic review and network analysis was conducted according to the standards of the Cochrane Handbook and the PRISMA Statement (PROSPERO registration number: CRD42017057944) (10-12). Medline, Embase, and Cochrane Library electronic databases were comprehensively searched to identify all potential eligible studies from inception to Oct 31, 2018 (Table S1). Additionally, unpublished data were identified from the website of ClinicalTrials.gov. The bibliographies of published trials as well as the systematic reviews were also scrutinized to identify any potentially relevant articles. The searches of databases were conducted by two reviewers (YJ Zhang and N Wang) independently, and all disagreements were resolved in discussion with a third author (ZC Gu).

Full table
Study selection
To be eligible for inclusion, the design of the study had to be a randomized controlled trials (RCTs) with the population including adult PAH patients. In addition, treatment regime had to involve ERAs (bosentan, ambrisentan, or macitentan) and reported safety data including abnormal liver function, peripheral edema and anemia for ERAs and placebo. All study titles and abstracts were independently assessed by two reviewers (YJ Zhang, N Wang), and afterwards the full paper was examined for any relevant possibility according to the inclusion. For reducing bias, journals, names of authors, as well as the publication year of the papers were blinded to the two reviewers. All uncertainties or discrepancies were resolved by consulting with other investigators
Data extraction, quality evaluation and bias assessment
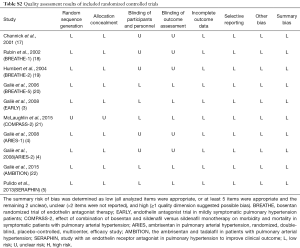
Two reviewers (YJ Zhang, N Wang) extracted the data independently with a standard form, presenting study population characteristics (first author’s name, publication year, sample size, mean age of the patients, sex ratio, WHO functional class, as well as the etiology of PAH), treatment and control groups, baseline therapy, time of duration, and other outcomes that were interested. Data not presented in the original publications was further extracted from the website of ClinicalTrials.gov. The quality of included RCTs was also evaluated employing the Cochrane Collaboration Risk of Bias Tool (13). The overall risk of bias was determined as low, unclear, or high according to the standards previously reported (10). Visually inspecting funnel plots was performed to evaluate bias of potential publication. The bias was considered minor when an approximate symmetrical funnel shape showed in the plot of the magnitude of treatment effect versus its precision estimate (14).
Data analysis
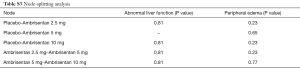
We used a network meta-analysis (NMA) by STATA software V.13 to carry out the direct and indirect comparison of treatments. Random-effects models were used to calculate risk ratios (RRs) and 95% confidence intervals (CI). And I2 values were used to evaluate to heterogeneity when at least two studies were available for each pairwise comparison, which defined as variation beyond chance (15). For inconsistency, we used a node-splitting analysis to evaluate whether direct and indirect evidence on the split node is in agreement (16). For ranking, a hierarchy of the treatments was provided using the surface under the cumulative ranking curve (SUCRA), which presents the overall ranking in a numeric way. Higher SUCRA value or closer to 100% represents that the therapy is in the top rank or one of the top ranks. Moreover, sensitivity analyses were performed to examine the effect by excluding RCTs that combined with other PAH-specific drugs in baseline therapy. Statistical significance was set at a P value <0.05, and all tests performed were two-sided.
Results
Study evaluation
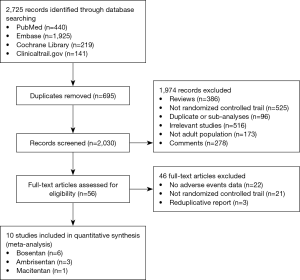
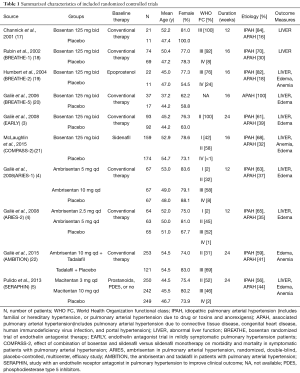
Totally, 2,725 studies were searched using the retrieval methods. After further review, 10 RCTs were included in the final analysis (3-5,17-22) (Figure 1, Table S1). Table 1 summarized the characteristics of included RCTs. Totally, 2,288 PAH patients were enrolled, of which 1,412 (61.7%) patients received ERAs and 876 (38.3%) patients received placebo. The average age of included patients was 50.7 years, 73.5% were females. Of these studies, 6 studies (780 patients) were bosentan, 3 studies (767 patients) were ambrisentan, and only 1 study (741 patients) was macitentan. Figure 2 showed the network map. The included studies had low bias overall (Table S2). Node-splitting analysis did not detect any inconsistency between direct evidence and indirect evidence (Table S3).
Full table

Full table
Full table
Direct comparisons without dose distinction
Results of direct comparison were shown in Table 2. As for abnormal liver function, bosentan showed a significantly higher risk (13.3% versus 4.5%; RR 2.93; 95% CI, 1.78–4.84; P<0.001) compared with placebo, whereas ambrisentan (0% versus 2.3%; RR 0.13; 95% CI, 0.01–1.13; P=0.06) and macitentan (3.5% versus 4.4%; RR 0.78; 95% CI, 0.37–1.64; P=0.52) did not show an increased risk. With regard to peripheral edema, ambrisentan showed a significantly higher risk compared with placebo (31.1% versus 19.0%; RR 1.62; 95% CI, 1.23–2.13; P=0.001), whereas bosentan (15.8% versus 11.9%; RR 1.32; 95% CI, 0.87−2.00; P=0.19) and macitentan (17.1% versus 18.1%; RR 0.95; 95% CI, 0.68−1.31; P=0.73) did not show an increased risk. Regarding anemia, macitentan was associated with a significantly higher risk compared with placebo (11.0% versus 3.2%; RR 3.42; 95% CI, 1.65−7.07; P<0.01), whereas no significant difference was observed for bosentan (8.3% versus 5.9%; RR 1.39; 95% CI, 0.67−2.86; P=0.37) and ambrisentan (14.6% versus 9.3%; RR 1.58; 95% CI, 0.88−2.82; P=0.12).
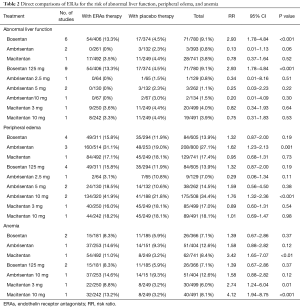
Full table
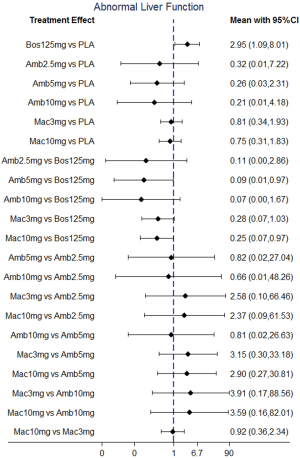
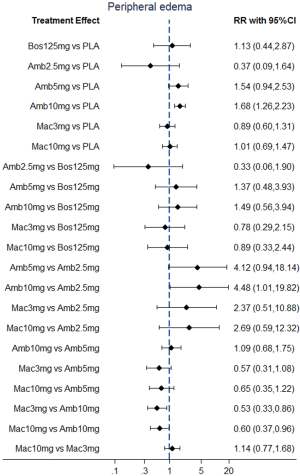
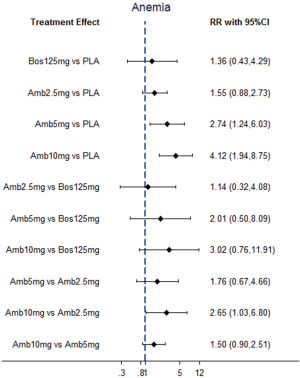
Direct comparisons and NMA with dose distinction
NMA results were presented in Figure 3. In terms of abnormal liver function, bosentan at 125 mg twice daily (NMA RR 2.56; 95% CI, 1.10−5.96) was significantly associated with a higher risk compared with placebo. The similar results were observed in the direct comparisons (Table 2). For peripheral edema, ambrisentan at 10 mg once daily (NMA RR 1.68; 95% CI, 1.26−2.23) showed a significantly higher risk versus placebo. The above finding was similar to the result of direct comparison (Table 2). In addition, Macitentan at dosages of 3 mg (NMA RR 0.53; 95% CI, 0.33−0.86) and 10 mg once daily (NMA RR 0.60; 95% CI, 0.37−0.96) were significantly associated with a lower risk when compared to ambrisentan at 10 mg once daily. Regarding anemia, macitentan at 3 mg (NMA RR 2.74; 95% CI, 1.24−6.03) and 10mg once daily (NMA RR 4.12; 95% CI, 1.94−8.75) showed a significantly higher risk compared with placebo. The similar results were observed in the direct comparisons (Table 2). A significantly higher risk of anemia was detected for macitentan at 10 mg once daily group versus bosentan at 125 mg twice daily group (NMA RR 2.90; 95% CI, 1.09−7.73) or versus ambrisentan at 10 mg once daily group (NMA RR 2.61; 95% CI, 1.01−6.76). No significant difference was observed for any other comparisons.
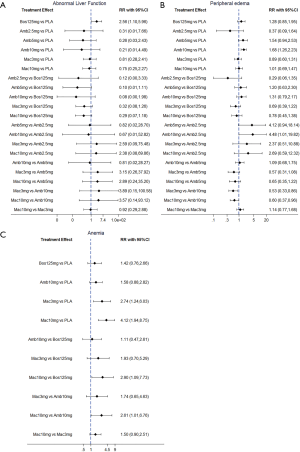
Rank probability
The SUCRA and absolute rank probabilities of ERAs were shown in Table 3. Bosentan at 125 mg twice daily had the highest risk of abnormal liver function, with a probability of 95.0%. With respect to peripheral edema, ambrisentan at 10 mg once daily had 52.9% probability with the highest risk among all treatments. As for anemia, macitentan at 10 mg once daily increased the risk most with probabilities of 90.8%.
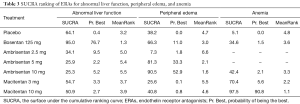
Full table
Sensitivity analyses and publication bias
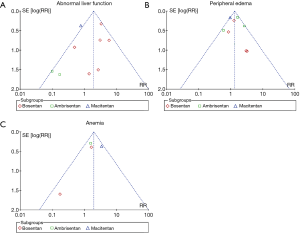
Sensitivity analysis was performed to confirm the robustness of the reported data. The analysis was repeatedly conducted after removing the RCTs that used a combination of other PAH-specific drugs in baseline therapy (19,22). The result did not change when removed 2 trials in the network (Tables S4,S5, Figures S1-S3). Visual inspection of funnel plots presented moderate symmetry, providing little evidence of publication bias (Figure S4).
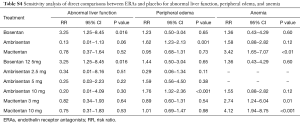
Full table

Full table
Discussion
PAH is a progressive disease characterized by the increased afterload and work of right ventricle, which result in right heart failure ultimately. Liver damage, edema, and anemia might be the indication of right cardiac failure and worsening PAH. In clinical practice, it is difficult to distinguish the various etiologies of these clinical adverse effects in PAH patients. These adverse effects could result from worsening right heart function, side effects of co-administrated drugs, or inadequate diuretic treatment. Even if the efficacy of ERAs is maintained, the development of these ERAs-relevant side effects could lead to poor tolerance of patients. This systematic review, based on network analysis, is the first to pool current safety evidence of ERAs in PAH patients, which could strengthen the evidence in head-to-head comparisons among different ERAs and help to guide clinical decision-making. We made several important observations in this network analysis. Firstly, compared with placebo, a significantly higher risk of abnormal liver function was observed in bosentan, and the highest risk presented in the dosage of 125 mg twice daily. Secondly, ambrisentan showed a significantly higher risk of peripheral edema compared with placebo, and ambrisentan at 10 mg once daily had the highest risk. Thirdly, macitentan might induce a significantly higher risk of anemia than placebo, and the highest risk was found at the dosage of 10 mg once daily. The latter evidence may inform choice of optimal ERAs treatment based on predicted safety property.
Abnormal liver function
To date, the exact mechanism of ERA-induced liver transaminitis has not been fully identified. Previous studies showed that it was likely to involve modulation of various hepatobiliary transporters, affinity for the ETB receptor, specific hepatic metabolic, and clearance pathways (23). In vitro studies, inhibition of uptake transporters including basolateral sodium-taurocholate co-transporting polypeptide (NTCP), organic anion transporting polypeptides (OATPs), efflux transporters bile salt export pump (BSEP), as well as multidrug resistance-associated protein 2 (MRP2) was observed in bosentan (24). Alterations in the activity of these proteins could result in cytotoxic bile acids accumulation (24,25). Of these transporters, BSEP, expressing at the canalicular domain of hepatocytes, has emerged as a probable protein for the development of drug-induced liver damage (25). In addition to BSEP, bosentan-relevant liver transaminitis might owe to its inhibition of ETB receptors on hepatocytes (26). Moreover, the elimination process of bosentan in human is entirely dependent on metabolism mediated by cytochrome P450 isoenzymes CYP2C9 and CYP3A4 in the liver (27). Thus, CYP2C9 polymorphism may be a marker for prediction of bosentan-induced liver injury due to poor metabolism (27). The Endothelin Antagonist Trial in Mildly Symptomatic PAH patients (EARLY) study of bosentan showed that elevation of liver enzymes was one of the most common cause of discontinued treatment (28). Importantly, elevation of alanine and aspartate aminotransferases (ALT/AST) was most commonly found more than 3 to 5× upper limit of normal (ULN), which remained unresolved, or dose reduced, or drug discontinued (28). In contrast, ambrisentan presented a weak inhibition of NTCP and OATPs, with little inhibition of BSEP (24).
In addition, the metabolic pathways of ambrisentan involved uridine 5’-diphosphate glucuronosyltransferases in a great degree, and CYP3A and CYP2C19 to a less extent, and almost entirely bile excretion for metabolites, which may partially explain the relatively low risk of hepatotoxicity in our finding (29). In fact, ambrisentan could be a safe substitution for patients with ERAs-induced transaminitis. In a phase II open-label study, ambrisentan were given to 36 PAH patients who had discontinued either bosentan or sitaxsentan due to liver enzymes elevation, and no cases of elevated aminotransferase levels were ultimately reported (30). In vitro, greater inhibition of NTCP, OATPs and BSEP was found in macitentan than bosentan (24). It is interesting that with the similar chemical structures and affinity to ETB receptor (31), different hepatotoxicity was observed in macitentan and bosentan in our finding. This difference may be the result of discrete changes in chemical structure. Replacing the sulfonamide with a sulfamide moiety that less acidic and increasing the lipophilicity might bring out a great change in the profile of hepatic disposition (31). Different from bosentan, most macitentan enters the liver through passive diffusion. Therefore, the extensive binding of macitentan to plasma proteins could limited the regional drug concentrations, making it difficult to play an inhibitory effect on hepatic bile salts (31,32). Accordingly, the pharmacokinetics of macitentan and its active metabolite ACT-132577 are similar in both hepatic impairment and healthy subjects, which implied the safety of macitentan even in patients with liver damage (33).
Peripheral edema
Peripheral edema is considered the most common adverse reaction in PAH therapies using ERAs, which might limit their use and effectiveness (8). In the present study, an increased risk of peripheral edema was only found in ambrisentan at the dosage of 10 mg once daily. The mechanism of ERAs-induced peripheral edema could be explained by cardiac, renal, or vascular effects. In a study, bosentan has been shown to decrease the contractility of the hypertrophied right ventricle by blocking ET-A receptors (34). Whereas, in a recent post hoc subgroup analysis, a reduction of brain natriuretic peptide (P<0.001) occurred in patients taking ambrisentan and with edema as compared to placebo (8). This suggested that the presence of peripheral edema is unlikely caused by cardiac dysfunction. Meanwhile, the use of ERAs could block natriuresis and diuresis through both ET-B receptors and ET-A receptors in the renal collecting ducts, as a result, causing fluid retention (8). Moreover, unopposed precapillary arteriolar vasodilation and changes in capillary permeability could also be included in the additional mechanisms (8).
In RCTs of ambrisentan in PAH (ARIES 1 and ARIES 2 trials), peripheral edema occurred incrementally in ambrisentan at the dosages of 2.5 mg, 5 mg and10 mg (3.1%, 9.5%, and 28.4%, respectively) (4). The data suggested that ambrisentan-induced edema appear to be a dose-related effect. Our analysis revealed that ambrisentan at 10 mg once daily showed a trend significantly increased risk of peripheral edema when compared to ambrisentan at 2.5 mg once daily, yielding an RR of 4.48 (95% CI, 1.01−19.82; P=0.07). Peripheral edema, with mild to moderate in severity, commonly continued to be an adverse reaction in ARIES extension study after open-label (35). Unlike ambrisentan, bosentan and macitentan showed a relatively low risk for peripheral edema and no patient required treatment discontinuation due to peripheral edema (5).
Anemia
For anemia, our analysis showed that macitentan had an increased risk, and 10 mg hold the highest risk. The mechanism underlying ERAs-induced anemia is not yet understood, however, it is thought to be partly secondary to increased fluid retention (10). In the study with an ERA in PAH to improve clinical outcome (SERAPHIN) trial, the incidence of anemia was found 8.8%, 13.2%, and 3.2% in macitentan at 3 mg once daily, 10 mg once daily, and placebo groups, respectively (5). It is appeared to be a dose-dependent effect of macitentan treatment. In contrast, bosentan and ambrisentan conferred relatively low risk of anemia.
Clinical implications
Taken together, the current evidence from RCTs and their meta-analysis suggest a variability across ERAs when regarding safety property, with a concern for bosentan in liver function, for ambrisentan in peripheral edema, and for macitentan in hemoglobin. In the clinical practice, different monitoring parameters should be considered for individual ERA in PAH. Patients taking bosentan, especially at the dosage of 125 mg twice daily, should undergo more hepatic monitoring. Peripheral edema warrants attention for patients receiving high-dose ambrisentan (10 mg once daily). Patients receiving macitentan, especially with the dosage of 10 mg once daily, is worthy of clinical monitoring in hemoglobin.
Limitations
Several important limitations are worth mentioning. First, only trials in English were enrolled, which may lead to potential bias of publication and selection. Second, the data was not evaluated based on various etiology of PAH or World Health Organization Functional Class (WHO FC). Accordingly, it is unavailable to make statistical powerful subgroup. Third, different baseline medication of PAH patients could also be an unignored factor to our results. Fourth, the duration of follow-up of the trials included in this meta-analysis varied from 12 to 56 weeks, which to some extent could influence the results. Additionally, none of the trials included in this study was specially designed to evaluate the safety of ERAs in PAH therapy. Accordingly, further design of RCTs focused on ERAs safety in PAH patients with a long-term duration of follow-up based on real-world experience is necessary.
Conclusions
Bosentan might be relevant to a higher risk of liver enzymes elevation; ambrisentan conferred a higher risk of peripheral edema; and macitentan increased a patient’s risk of anemia. In real-world practice, different monitoring parameters should be considered for different ERAs in PAH. Further head-to-head comparisons need to confirm these findings.
Acknowledgments
Funding: This work was supported by Research Funds of Shanghai health and family planning commission (20184Y0022), and Program for Key but Weak Discipline of Shanghai Municipal Commission of Health and Family Planning (2016ZB0304). Clinical Pharmacy Innovation Research Institute of Shanghai Jiao Tong University School of Medicine (CXYJY2019ZD001), and the National Nature Science Foundation of China (No. 81803841).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This systematic review and network analysis was reported in accordance with standards outlined in the Cochrane Handbook and the PRISMA Extension Statement (PROSPERO registration number: CRD42017057944).
References
- Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest 2014;146:449-75. [Crossref] [PubMed]
- Lajoie AC, Lauziere G, Lega JC, et al. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med 2016;4:291-305. [Crossref] [PubMed]
- Galiè N, Rubin Lj, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 2008;371:2093-100. [Crossref] [PubMed]
- Galiè N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008;117:3010-9. [Crossref] [PubMed]
- Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013;369:809-18. [Crossref] [PubMed]
- Segal ES, Valette C, Oster L, et al. Risk management strategies in the postmarketing period: safety experience with the US and European bosentan surveillance programmes. Drug Saf 2005;28:971-80. [Crossref] [PubMed]
- Ben-Yehuda O, Pizzuti D, Brown A, et al. Long-term hepatic safety of ambrisentan in patients with pulmonary arterial hypertension. J Am Coll Cardiol 2012;60:80-1. [Crossref] [PubMed]
- Shapiro S, Pollock DM, Gillies H, et al. Frequency of edema in patients with pulmonary arterial hypertension receiving ambrisentan. Am J Cardiol 2012;110:1373-7. [Crossref] [PubMed]
- Formulary watch. Pfizer voluntarily withdraws sitaxsentan from the market worldwide and halts ongoing clinical trials. 2011. Accessed 31 Oct 2017. Available online: https://www.formularywatch.com/cardiovascular/pfizer-voluntarily-withdraws-sitaxsentan-market-worldwide-and-halts-ongoing-clinical-trials
- Wei A, Gu Z, Li J, et al. Clinical Adverse Effects of Endothelin Receptor Antagonists: Insights From the Meta-Analysis of 4894 Patients From 24 Randomized Double-Blind Placebo-Controlled Clinical Trials. J Am Heart Assoc 2016;5:e003896. [Crossref] [PubMed]
- Higgins JPT, Green S. editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration, 2011.
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777-84. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Wei AH, Gu ZC, Zhang C, et al. Increased risk of myocardial infarction with dabigatran etexilate: fact or fiction? A critical meta-analysis of over 580,000 patients from integrating randomized controlled trials and real-world studies. Int J Cardiol 2018;267:1-7. [Crossref] [PubMed]
- Gu ZC, Zhou LY, Shen L, et al. Non-vitamin K Antagonist Oral Anticoagulants vs. Warfarin at Risk of Fractures: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Pharmacol 2018;9:348. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet 2001;358:1119-23. [Crossref] [PubMed]
- Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002;346:896-903. [Crossref] [PubMed]
- Humbert M, Barst RJ, Robbins IM, et al. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J 2004;24:353-9. [Crossref] [PubMed]
- Galiè N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006;114:48-54. [Crossref] [PubMed]
- McLaughlin V, Channick RN, Ghofrani HA, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J 2015;46:405-13. [Crossref] [PubMed]
- Galiè N, Barberà JA, Frost AE, et al. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N Engl J Med 2015;373:834-44. [Crossref] [PubMed]
- Aversa M, Porter S, Granton J. Comparative safety and tolerability of endothelin receptor antagonists in pulmonary arterial hypertension. Drug Saf 2015;38:419-35. [Crossref] [PubMed]
- Lepist EI, Gillies H, Smith W, et al. Evaluation of the endothelin receptor antagonists ambrisentan, bosentan, macitentan, and sitaxsentan as hepatobiliary transporter inhibitors and substrates in sandwich-cultured human hepatocytes. PLoS One 2014;9:e87548. [Crossref] [PubMed]
- Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 2001;69:223-31. [Crossref] [PubMed]
- Ling L, Kuc RE, Maguire JJ, et al. Comparison of endothelin receptors in normal versus cirrhotic human liver and in the liver from endothelial cell-specific ETB knockout mice. Life Sci 2012;91:716-22. [Crossref] [PubMed]
- Markova SM, De Marco T, Bendjilali N, et al. Association of CYP2C9*2 with bosentan-induced liver injury. Clin Pharmacol Ther 2013;94:678-86. [Crossref] [PubMed]
- Simonneau G, Galie N, Jansa P, et al. Long-term results from the EARLY study of bosentan in WHO functional class II pulmonary arterial hypertension patients. Int J Cardiol 2014;172:332-9. [Crossref] [PubMed]
- Buckley MS, Wicks LM, Staib RL, et al. Pharmacokinetic evaluation of ambrisentan. Expert Opin Drug Metab Toxicol 2011;7:371-80. [Crossref] [PubMed]
- McGoon MD, Frost AE, Oudiz RJ, et al. Ambrisentan therapy in patients with pulmonary arterial hypertension who discontinued bosentan or sitaxsentan due to liver function test abnormalities. Chest 2009;135:122-9. [Crossref] [PubMed]
- Sidharta PN, Krahenbuhl S, Dingemanse J. Pharmacokinetic and pharmacodynamic evaluation of macitentan, a novel endothelin receptor antagonist for the treatment of pulmonary arterial hypertension. Expert Opin Drug Metab Toxicol 2015;11:437-49. [Crossref] [PubMed]
- Treiber A, Aanismaa P, de Kanter R, et al. Macitentan does not interfere with hepatic bile salt transport. J Pharmacol Exp Ther 2014;350:130-43. [Crossref] [PubMed]
- Sidharta PN, Lindegger N, Ulc I, et al. Pharmacokinetics of the novel dual endothelin receptor antagonist macitentan in subjects with hepatic or renal impairment. J Clin Pharmacol 2014;54:291-300. [Crossref] [PubMed]
- Nagendran J, Sutendra G, Paterson I, et al. Endothelin axis is upregulated in human and rat right ventricular hypertrophy. Circ Res 2013;112:347-54. [Crossref] [PubMed]
- Oudiz RJ, Galie N, Olschewski H, et al. Long-term ambrisentan therapy for the treatment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:1971-81. [Crossref] [PubMed]