
Effect of paeoniflorin on cardiac remodeling in chronic heart failure rats through the transforming growth factor β1/Smad signaling pathway
Introduction
Chronic heart failure (CHF) is a complex clinical syndrome caused by various cardiovascular diseases. In CHF patients, the cardiac structure and function are found abnormal and the ventricular filling and/or ejection capacity are reduced (1). The prevalence of heart failure in adult population is about 1–2% in developed countries. Cardiac remodeling is an important mechanism for the occurrence and development of CHF. It can reduce myocardial compliance and contractility (2). In recent years, studies have reported that immune-inflammatory activation and sustained myocardial inflammation may play an important role in the development of cardiac remodeling (3-5). Previous studies have found that methotrexate, rapamycin and triptolide can regulate inflammatory responses, alleviate cardiac fibrosis and improve cardiac function in rats (6-8). Therefore, more and more scholars all over the world began to explore the application value of anti-inflammatory and immunomodulation strategy in CHF.
Paeoniflorin (Pae) is a monoterpenoid glycoside compound and is the main active ingredient of Paeonia lactiflora (9). It has many biological activities, such as immunosuppression, anti-inflammatory and anti-cell proliferation (10,11). Besides, Pae can improve renal, lung and liver fibrosis by inhibiting the expression of transforming growth factor β1 (TGF-β1) and reducing the level of inflammatory factors (12,13). However, the effect and underlying mechanism of remains unclear. In the present study, we hypothesized that Pae alleviates cardiac remodeling and improves cardiac function in isoprenaline (Iso) induced CHF rats. Therefore, this study was conducted to examine the hypothesis.
Methods
Animal
A total of thirty-two male SPF Wistar rats (SCXK YUE 2011-0029), aged 8-week-old, weighting 200–230 g, were provided by Laboratory Animals Center of Sun Yat-sen University (Guangzhou, China). The rats were acclimatized to the laboratory environment and allowed free access to food and water for 3 days prior to the experiments.
Material
Masson’s Trichrome Stain Kit was purchased from PanEra Co. Ltd (Guangzhou, China). Anti-collagen type I antibody were purchased from Boster Co. Ltd (Wuhan, China). BCA Protein Assay kit was purchased from KGI Biological Technology Co. Ltd (Jiangsu, China). Trizol was purchased from Invitrogen (CA, USA). Primers were provided by GENEWIZ Co. Ltd (NJ, USA). Synthesis Kit of the first-strand cDNA and RT-PCR Kit were purchased from GeneCopoeia (MD, USA). Hydroxyproline (HYP) assay kit was obtained from Jiancheng Bioengineering Institute (Nanjing, China). Iso was purchased from Boyuan Pharmaceutical Co. Ltd (Jinan, China). Pae was purchased from Gold Wheat biology Co. Ltd (Shanghai, China). Captopril was purchased from Jinshi General Pharmaceutical Factory (Shantou, China). Other materials are all analytical reagents.
Grouping and modeling
The rats were numbered by weight before modeling. Eight rats were randomly selected as vehicle control group (Veh). To establish the cardiac remodeling model, rats were administered multiple back injections of 5 mg/(kg·d) Iso per 24 hours for 10 days. In the next six weeks after modelling, the CHF rats were randomly divided into three groups: Iso, Pae and Cap group. In the Iso group, rats were given dimethyl sulfoxide (DMSO) (1 mL/100 g/d) daily. Pae was dissolved with DMSO. The concentration of Pae was 2.5 mg/L. Rats in Pae group were administered with Pae [20 µg/(kg·d)] daily. Rats in Cap group were administered with Cap [15 mg/(kg·d)] daily. Rats in Veh group was administered DMSO (1 mL/100 g/d) daily.
Echocardiography
The rats were subsequently fasted in 24 hr after the last administration. The rats were anaesthetized by intraperitoneal injection with 2% pentobarbital sodium (50 mg/kg). Echocardiography was used to examine the cardiac function and structure. Three images containing consecutive cardiac cycle at each position were saved. Left ventricular internal diameter at end-systole (LVIDs), left ventricular internal diameter at end-diastole (LVIDd), fractional shortening fraction (FS) and ejection fraction (EF) were measured.
Histopathology
The rat ventricular samples were fixed in 10% formalin, embedded in paraffin and cut into sections. The sections were dyed with Masson’s trichrome stain kit to evaluate the myocardial fibrosis. Immunohistochemistry was also used to assess the collagen I expression in myocardial tissue. The collagen I presents brown fine particles under a microscope if the immunohistochemistry is positive.
Collagen volume fraction (CVF) and perivascular collagen area (PVCA)
To evaluate severity of cardiac remodeling, Masson staining slices were taken to calculate CVF and PVCA by using Image-Pro Plus 6.0 image analysis system. CVF = collagen area/total area. PVCA = collagen area around the arterial lumen/arterial lumen area. Six visions under microscope of each sample were randomly chosen. The histological specimens were observed under microscope (ZEISS Axio Scope A1, Oberkochen, Germany). Image Pro Plus 6.0 software (Media Cybernetics, MD, USA) was used for image analysis (14).
HYP concentration
About 30–100 mg ventricular tissue was cut and centrifuged. The tissue was added into 1ml hydrolytic agent in 95 °C constant temperature bath for 20 min. The PH was adjusted to 6.0–6.8. HYP kit was used to detect the content of HYP. HYP concentration in ventricular tissue (µg/mg) = (sample tube OD-blank tube OD) × standard sample concentration (5 µg/mL) × total volume (10 mL)/[(standard tube OD − blank tube OD) × tissue wet weight (mg)].
Western blot
Western blots were used to quantify the protein levels of TGF-β1 and Smad3. Standard curve was made to quantify protein sample preliminarily according to the manual of BCA assay kit. And then protein solution was mixed with pre-prepared 5× loading buffer. The mixture was separated using gel electrophoresis until bromophenol blue moved out. PVDF membrane of methanol pretreatment was put in transfer liquid and infiltrated. Gel was placed on the filter, forming “sandwich” structure of gel transferring stacking layer. Horseradish peroxidase-conjugated anti-immunoglobulin G was used as a secondary antibody. Tanon multi-function gel imaging system was used for quantitative analysis.
Real-time PCR
Myocardial tissue was ground with liquid nitrogen and then 1 mL Trizol was added in and mixed. As a result, the total RNA had been extracted. Genomic DNA degraded using RNase-free DNase I before detection of the purity and integrity of the total RNA. Dissolve the first chain cDNA and put it on the ice. Base sequences of primers are in the following table (Table 1). Each experiment was performed at least three times. In this study, β-actin was used as an internal control gene to normalize the mRNA levels of TGF-β1 and Smad3 in ventricular tissues. The test was repeated 3 times and the results were averaged.
Full table
Statistical analysis
In this study, the quantitative data were presented as mean ± standard deviation. Normality test and homogeneity test of variance were used. One-way ANOVA was used for the multi-independent sample. While Kruskal-Wallis test was performed if non-normal distribution or heterogeneity of variance was found. A two-tale P<0.05 was considered to indicate a significant difference. All data were analyzed with IBM SPSS software Version 20.0 for Windows (SPSS Inc., Chicago, USA).
Results
Cardiac structure and function
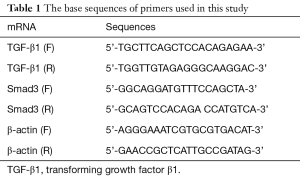
As shown in Figure 1, compared to Veh group, rats in Iso group seemed to be apathetic and fatigued. Cardiomegaly was also found in the Iso group. Furthermore, LVIDd and LVIDs were elevated. EF and FS were also decreased significantly in Iso group. However, compared to Iso group, LVIDd (6.12±0.32 vs. 8.40±0.52 mm, P=0.002) and LVIDs (4.16±0.19 vs. 6.44±0.93 mm, P=0.001) in Pae group had been decreased. EF (73.67%±4.41% vs. 46.44%±3.50%, P<0.001) and FS (36.44%±2.34% vs. 23.00%±2.08%, P<0.001) increased significantly. Besides, LVIDd (6.57±0.41 vs. 8.40±0.52 mm, P=0.005) and LVIDs (4.82±0.28 vs. 6.44±0.93 mm, P=0.005) were also found decreased in Cap group. Amelioration of EF (63.44%±1.64% vs. 46.44%±3.50%, P=0.001) and FS (32.00%±1.45% vs. 23.00%±2.08%, P=0.003) were also found in Cap group in the comparison with Iso group (Figure S1, Table S1).

Full table
VB/WB ratio
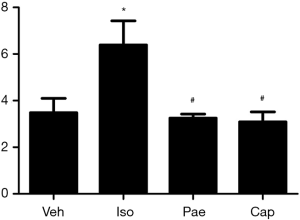
As shown in Figure 2, higher VW/BW ratio was found in Iso group (3.48±0.62 vs. 6.39±1.03 mg/g, P<0.001) compared with Veh group. Compared to Iso group, Pae (3.25±0.17 vs. 6.39±1.03 mg/g, P<0.001) or Cap (3.09±0.43 vs. 6.39±1.03 mg/g, P<0.001) treatment could reduce the VW/BW ratios markedly.
HE and Masson staining
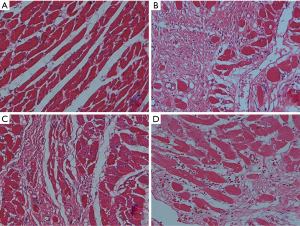
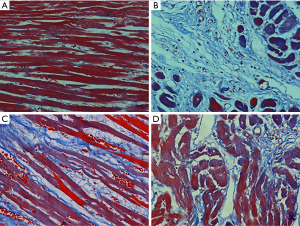
As shown in Figure 3, in the HE staining, the myocardial tissues of Veh group were arranged in order. Myocardial tissues was arranged irregularly and infiltrated with inflammatory cells in the Iso group. Pae or Cap treatment could ameliorate the myocardial injury in CHF rats. Besides, as shown in Figure 4, in the Masson staining, there were few stained collagen fibers in Veh group. Collagen fibers in the Iso group were markedly increased compared with normal controlled group, especially in subendocardial myocardium and perivascular areas. Compared to Iso group, Pae or Cap treatment can significantly reduce the expression of collagen fibers and ameliorate the myocardial injury in myocardial tissues.
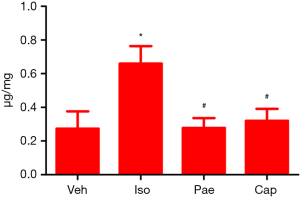
HYP concentration
As shown in Figure 5, compared to Veh group, the HYP concentration was increased markedly in Iso group (0.27±0.10 vs. 0.66±0.06 µg/mg, P=0.001). Pae (0.66±0.10 vs. 0.28±0.06 µg/mg, P<0.001) or Cap (0.66±0.10 vs. 0.32±0.07 µg/mg, P=0.014) treatment can decrease the HYP concentration in myocardial tissue significantly compared with the Iso group.
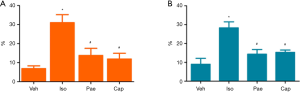
CVF and PVCA
As shown in Figure 6, compared to Veh group, CVF (6.85%±1.48% vs. 30.97%±4.22%, P<0.001) and PVCA (8.97%±3.16% vs. 28.31%±3.16%, P<0.001) of myocardial tissues were increased in Iso group. The treatment with Pae can decrease the CVF (13.75%±3.77% vs. 30.97%±4.22%, P<0.001) and PVCA (14.32%±2.50% vs. 28.31%±3.16%, P<0.001) significantly (P<0.001). Besides, CVF (11.90%±3.01% vs. 30.97%±4.22%, P<0.001) and PVCA (15.27%±1.24% vs. 28.31%±3.16%, P<0.001) were also decreased in Cap group, compared with Iso group.
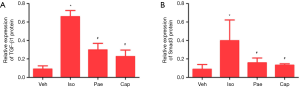
Effect of Pae on protein level of TGF-â1 and Smad3
According to the results in Figure 7, the protein levels of TGF-β1 (0.09±0.04 vs. 0.66±0.07, P<0.001) and Smad3 (0.09±0.05 vs. 0.40±0.22, P=0.012) were significantly increased in Iso group compared with the Veh group. Compared to Iso group, the protein levels of TGF-β1 (0.30±0.07 vs. 0.66±0.07, P<0.001) and Smad3 (0.16±0.05 vs. 0.40±0.22, P=0.037) in Pae group had been decreased in CHF rats. The protein levels of TGF-β1 (0.23±0.07 vs. 0.66±0.07, P<0.001) and Smad3 (0.13±0.02 vs. 0.40±0.22, P=0.025) in Cap group were also lower than Iso group.
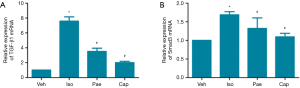
Effect of Pae on relative expression of TGF-â1 mRNA and Smad3 mRNA
As shown in Figure 8, compared to Veh group, the relative expression of TGF-β1 and Smad3 mRNA in myocardial tissues were increased in Iso group (P<0.001). Compared to Iso group, the relative expressions of TGF-β1 mRNA were decreased significantly in Pae group (3.51±0.44 vs. 7.58±0.58, P<0.001) and Cap group (2.01±0.18 vs. 7.58±0.58, P<0.001). Also, the relative expressions of Smad3 mRNA were lower than Iso group in Pae (1.32±0.28 vs. 1.69±0.08, P=0.047) or Cap (1.09±0.09 vs. 1.69±0.08, P=0.047) treated rats.
Discussion
In this study, Pae was administrated to Iso induced CHF rats for 6 weeks. Compared to Iso group, Pae can alleviate cardiac remodeling and improve cardiac function. The levels of CVF and PVCA reduced in Pae and Cap group. The expression of TGF-β1 and Smad3 proteins decreased in Pae and Cap group. The relative expression of mRNA of TGF-β1 and Smad3 also decreased markedly in Pae and Cap group.
CHF, the severe and end stage of a variety of heart diseases, is one of the most important cardiovascular diseases in the 21st century and seriously threatens people's health and life all over the world (1). Cardiac remodeling is the basic mechanism of CHF (3). Myocardial inflammation and immune disorder have been reported to be related to the development of cardiac remodeling (5,15). Therefore, anti-inflammatory and immunomodulation has become one of the research hot spots in the prevention and treatment of CHF (5,6).
Pae is a monoterpene glycoside compounds with immunosuppressive, anti-inflammatory and anti-proliferation activities. It is the main active ingredient of traditional Chinese medicine peony (16-18). Recent studies have found that Pae can inhibit the secretion of HIF-1α through mTOR signaling pathway, reduce the expression of α-SMA and collagen protein and relieve liver fibrosis (19). However, the effects of Pae in CHF remain unclear.
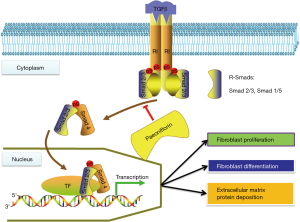
In this study, Pae and Cap were given in CHF rats for 6 weeks. It was found that Pae and Cap could alleviate cardiac remodeling, reduce LVIDd, LVIDs, CVF and PVCA, and improve cardiac function in CHF rats. The effect of Pae on cardiac remodeling found in this study was similar to other reports in hypertensive rats (20,21). Meanwhile, in order to explore the potential mechanism of Pae on cardiac remodeling, this study examined the effect of Pae on the transcription and translation of TGF-β1 and Smad3. As we know, TGF-β1/Smads signaling pathway is the most important pathway for organ fibrosis (6,22). In this study, the expression of the protein and mRNA of TGF-β1 and Smad3 were found decreased markedly in the Pae treated group, indicating that Pae may reverse cardiac remodeling in CHF rats via inhibiting the TGF-β1/Smad pathway (Figure S2). Therefore, this study has provide basic research proof for the mechanism of Pae through TGF-β1/Smad pathway on cardiac remodeling in CHF rats.
Conclusions
The results indicated that Pae could reduce the expression of HYP and TGF-β1, reduce cardiac remodeling and improve cardiac function in CHF rats. The cardio-protective effect of Pae may be associated with the inhibition of TGF-β1/Smad pathways.
Acknowledgments
Funding: The study was funded by Scientific Research Development Program of North Sichuan Medical College (CBY16-A-ZD10) and Nanchong Government-University Strategic Cooperation Project in Science and Technology (18SXHZ0505).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our experimental study was approved by the School Animal Experimental Ethics Committee.
References
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Schirone L, Forte M, Palmerio S, et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxid Med Cell Longev 2017;2017:3920195. [Crossref] [PubMed]
- Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014;71:549-74. [Crossref] [PubMed]
- Al-Darraji A, Haydar D, Chelvarajan L, et al. Azithromycin therapy reduces cardiac inflammation and mitigates adverse cardiac remodeling after myocardial infarction: Potential therapeutic targets in ischemic heart disease. PLoS One 2018;13:e0200474. [Crossref] [PubMed]
- Zhang Y, Bauersachs J, Langer HF. Immune mechanisms in heart failure. Eur J Heart Fail 2017;19:1379-89. [Crossref] [PubMed]
- Liu M, Chen J, Huang Y, et al. Triptolide alleviates isoprenaline-induced cardiac remodeling in rats via TGF-beta1/Smad3 and p38 MAPK signaling pathway. Pharmazie 2015;70:244-50. [PubMed]
- Maranhão RC, Guido MC, de Lima AD, et al. Methotrexate carried in lipid core nanoparticles reduces myocardial infarction size and improves cardiac function in rats. Int J Nanomedicine 2017;12:3767-84. [Crossref] [PubMed]
- Yu S Y, Liu L, Li P, et al. Rapamycin inhibits the mTOR/p70S6K pathway and attenuates cardiac fibrosis in adriamycin-induced dilated cardiomyopathy. Thorac Cardiovasc Surg 2013;61:223-8. [PubMed]
- Abd El-Aal NF, Abdelbary EH. Paeoniflorin in experimental BALB/c mansoniasis: A novel anti-angiogenic therapy. Exp Parasitol 2019;197:85-92. [Crossref] [PubMed]
- Ji Y, Wang T, Wei ZF, et al. Paeoniflorin, the main active constituent of Paeonia lactiflora roots, attenuates bleomycin-induced pulmonary fibrosis in mice by suppressing the synthesis of type I collagen. J Ethnopharmacol 2013;149:825-32. [Crossref] [PubMed]
- Chen H, Dong Y, He X, et al. Paeoniflorin improves cardiac function and decreases adverse postinfarction left ventricular remodeling in a rat model of acute myocardial infarction. Drug Des Devel Ther 2018;12:823-36. [Crossref] [PubMed]
- Shao YX, Xu XX, Wang K, et al. Paeoniflorin attenuates incipient diabetic nephropathy in streptozotocin-induced mice by the suppression of the Toll-like receptor-2 signaling pathway. Drug Des Devel Ther 2017;11:3221-33. [Crossref] [PubMed]
- Ji Y, Dou YN, Zhao QW, et al. Paeoniflorin suppresses TGF-beta mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol Sin 2016;37:794-804. [Crossref] [PubMed]
- Liu M, Chen J, Yao J, et al. Triptolide Attenuates Cardiac Remodeling in Isoprenaline-induced Chronic Heart Failure Rats via Upregulating PTEN Pathway. Journal of Sun Yat-sen University Medical Sciences 2017;38:29-35.
- Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res 2016;119:91-112. [Crossref] [PubMed]
- Li H, Jiao Y, Xie M. Paeoniflorin Ameliorates Atherosclerosis by Suppressing TLR4-Mediated NF-kappaB Activation. Inflammation 2017;40:2042-51. [Crossref] [PubMed]
- Wang Z, Liu Z, Yu G, et al. Paeoniflorin Inhibits Migration and Invasion of Human Glioblastoma Cells via Suppression Transforming Growth Factor beta-Induced Epithelial-Mesenchymal Transition. Neurochem Res 2018;43:760-74. [Crossref] [PubMed]
- Zhao L, Chang Q, Huang T, et al. Paeoniflorin inhibits IL1betainduced expression of inflammatory mediators in human osteoarthritic chondrocyte. Mol Med Rep 2018;17:3306-11. [PubMed]
- Zhao Y, Ma X, Wang J, et al. Paeoniflorin alleviates liver fibrosis by inhibiting HIF-1alpha through mTOR-dependent pathway. Fitoterapia 2014;99:318-27. [Crossref] [PubMed]
- Zhou H, Yang HX, Yuan Y, et al. Paeoniflorin attenuates pressure overload-induced cardiac remodeling via inhibition of TGFbeta/Smads and NF-kappaB pathways. J Mol Histol 2013;44:357-67. [Crossref] [PubMed]
- Liu X, Chen K, Zhuang Y, et al. Paeoniflorin improves pressure overload-induced cardiac remodeling by modulating the MAPK signaling pathway in spontaneously hypertensive rats. Biomed Pharmacother 2019;111:695-704. [Crossref] [PubMed]
- Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 2016;12:325-38. [Crossref] [PubMed]


