Progression of coronary atherosclerosis in African-American patients
Introduction
Increasing evidence demonstrates that African-American patients with coronary artery disease (CAD) experience a high rate of adverse cardiovascular events (1,2), becoming a major public health problem in the United States. The reasons underlying this disparity in clinical outcomes remain a source of ongoing investigation. Previous studies have reported that African-Americans exhibit a higher prevalence of major risk factors, including smoking, hypertension, diabetes and obesity, which may contribute to worse outcomes (3-5). These differences are related, in part, to diminished access to health care services and underutilization of preventive therapies (6-8). However, it remains to be determined whether other differences in disease pathophysiology may contribute to the clinical expression of the disease in African-Americans.
Mechanistic studies have demonstrated a high prevalence of endothelial dysfunction, a critical early event in atherogenesis, in African-Americans (9-11). It is unknown whether other molecular events implicated in atherosclerosis are upregulated. Furthermore, no systematic assessment of coronary atherosclerosis and its progression has been performed in African-Americans with arterial wall imaging.
Intravascular ultrasound (IVUS) imaging generates high resolution imaging of the full thickness of the artery wall, permitting precise quantitation of atheroma burden and progression on serial evaluation. This enables assessment of the effects of anti-atherosclerotic therapies and characterization of the clinical factors that influence disease progression (12-20). The objective of the current analysis was to characterize the burden and progression of coronary atherosclerosis in African-American patients with CAD.
Methods
Study population
The analysis pooled data from 7 clinical trials that assessed the impact of medical therapies on changes in coronary atheroma burden using IVUS (14-20). Each study enrolled patients with angiographic CAD, defined as the presence of a lumen stenosis greater than 20% in at least one major epicardial coronary artery on a clinically indicated coronary angiogram. Each study was approved by the institutional review boards of the participating clinical trial sites, and all participants provided informed written consent before enrollment.
Acquisition and analysis of IVUS images
The methods for acquisition and analysis of IVUS images have been described in detail previously (14-20). In brief, a target vessel without luminal stenosis greater than 50% in a segment of at least 30 mm in length was selected for imaging. The vessel was required to not have undergone previous revascularization or represent the culprit vessel for a prior myocardial infarction. After anticoagulation therapy and administration of intracoronary nitroglycerin, an imaging catheter containing a high-frequency ultrasound transducer (30-40 MHz) was inserted distally within the coronary artery. Ultrasonic images were continuously recorded on videotape during withdrawal of the catheter at a constant rate of 0.5 mm/s. Imaging was performed within the same coronary artery at baseline and at the end of the study, after 18-24 months of treatment.
The recorded images were digitized for subsequent analysis. An anatomically matched segment was defined at the 2 time points on the basis of proximal and distal side branches (fiduciary points). Cross-sectional images spaced precisely 1-mm apart were selected for measurement. The leading edges of the lumen and external elastic membrane (EEM) were traced by manual planimetry. Atheroma volume was calculated as the summation of plaque area, defined as the difference between EEM and lumen area, in each measured image, and then normalized to account for differences in segment length between different subjects:
Atheroma volume (mm3) = [Σ (EEMarea–Lumenarea)/number of slices in pullback] × median number of slices in study population
The percent atheroma volume was calculated as the proportion of vessel wall volume occupied by atherosclerotic plaque:
Percent atheroma volume (%) = [Σ (EEMarea–Lumenarea)/Σ EEMarea] ×100
Volumes occupied by the lumen and EEM were similarly calculated by summation of their respective areas in each measured image and subsequently normalized to account for differences in segment length between subjects.
Calcification was defined as the presence of high signal intensity with acoustic shadowing. Percentage of images containing calcification through the imaging segment was compared between African-American and Caucasian patients with CAD.
Statistical analysis
Clinical characteristics were presented as percentage for categorical variables and mean ± SD continuous variables or median (interquartile range) when continuous variables were not normally distributed. Groups were compared using two-sample t tests or Wilcoxon rank-sum tests for continuous variables and chi-square or Fisher’s exact tests for categorical variables, depending on the pattern of distribution of data. Mixed-effects models compared changes in atheroma volumes between groups, including baseline atheroma volume as a covariate and adjusting for trial differences. Medical history, concomitant medication use and lipid parameters were also considered for inclusion into the model and a stepwise elimination procedure was used to determine the final model. A propensity score for African-American patients was generated using logistic regression analysis, including all clinical variables and potential differences from Whites. Each African-American patient was subsequently matched with three Caucasians using the propensity score. The c-statistic for the propensity score was 0.81 indicating good discrimination between the two groups. A two-sided P value <0.05 was considered statistically significant. All statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Cary North Carolina).
Results
Baseline characteristics
In the current study, 4.8% of study population was African-American patients. Clinical characteristics of patients are summarized in Table 1. African-American patients were more likely to be female (51.8% vs. 28.1%, P<0.001) and have a higher body mass index (32.8±6.0 vs. 31.3±5.8 kg/m2, P=0.002), and have prevalent hypertension (85.9% vs. 78.8%, P=0.02), diabetes (41.8% vs. 30.6%, P=0.002) and prior stroke (12.9% vs. 3.0%, P<0.001) than Caucasian patients. Apart from a higher rate of use of calcium-channel blocker (42.4% vs. 33.1%, P=0.04), oral anti-diabetic agents (36.5% vs. 23.8%, P<0.001) and insulin (20.6% vs. 8.4%, P<0.001) in African-American patients, no other differences in medication use were observed between two groups. African-American patients demonstrated higher levels of high-density lipoprotein cholesterol (HDL-C: 1.2±0.3 vs. 1.1±0.3 mmol/L, P<0.001), c-reactive protein (CRP: 4.4 vs. 2.6 mg/dL, P<0.001), glycated hemoglobin (6.9%±1.4% vs. 6.4%±1.3%, P=0.002) and diastolic blood pressure (77±10 vs. 76±9 mmHg, P=0.03), and lower triglyceride levels (1.2 vs. 1.6 mmol/L, P<0.001) compared with Caucasians. Assigned treatments in each IVUS clinical trials were also summarized in Table 1. There were no significant differences in assigned medical therapies of each trial between African-American and Caucasian patients.
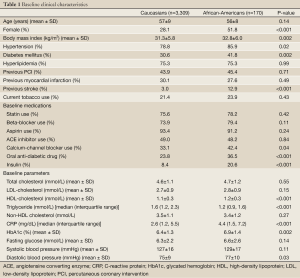
Full Table
Risk factor control
Concomitant medication use and risk factor control during the course of the trials are summarized in Table 2. African-American patients were less likely to be treated with aspirin (88.8% vs. 94.5%, P=0.002) and more likely to be treated with a calcium-channel antagonist (48.2% vs. 37.7%, P=0.006), oral anti-diabetic drug (43.5% vs. 28.0%, P<0.001) and insulin (19.4% vs. 9.3%, P<0.001). Despite frequent use of lipid and blood pressure lowering therapies in both groups (93% statin, 89% aspirin, 79% β-blocker, 52% ACE inhibitor), African-American patients demonstrated higher levels of low-density lipoprotein cholesterol (LDL-C: 2.4±0.7 vs. 2.2±0.7 mmol/L, P=0.006), CRP (2.9 vs. 2.0 mg/dL, P<0.001), systolic blood pressure (133±15 vs. 128±13 mmHg, P<0.001) and diastolic blood pressure (78±7 vs. 76±7 mmHg, P=0.001) compared with Caucasian patients. This translated to a lower proportion of African-American patients with more optimal lowering of LDL-C <2.1 mmol/L (34.1% vs. 42.8%, P=0.02), CRP <2.0 mg/dL (37.3% vs. 49.3%, P=0.004) and systolic blood pressure <130 mmHg (44.2% vs. 58.9%, P<0.001). In contrast, African-American patients demonstrated more favorable levels of HDL-C (1.3±0.4 vs. 1.2±0.4 mmol/L, P=0.001) and triglycerides (1.2 vs. 1.5 mmol/L, P<0.001).
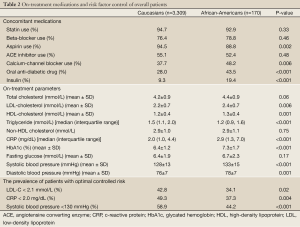
Full Table
Atherosclerosis burden and progression
Ultrasound-derived measures of atheroma burden and vascular dimensions at baseline and their serial changes are summarized in Tables 3,4, respectively. There were no significant differences in IVUS measures between the groups at baseline (Table 3). Propensity matching analysis also showed the same characteristics of coronary atherosclerosis in two groups (Table 3). On serial evaluation, a greater increase in atheroma volume was observed in African-American patients (+0.51±2.14 vs. –3.06±1.66 mm3, P=0.01). Subsequent propensity matching for clinical factors produced a comparison, whereby on-treatment systolic (133.1±14.5 vs. 129.2±12.3 mmHg, P=0.002) and diastolic (78.5±7.1 vs. 77.2±7.8 mmHg, P=0.05) blood pressure levels remained higher in African-American patients. Propensity analysis revealed that the greater increase in atheroma volume in African-American patients persisted (+0.13±2.10 vs. –3.71±1.69 mm3, P=0.02). Further adjustment for differences in baseline atheroma volume, risk factors, medication use, lipid and blood pressure levels still demonstrated greater progression of atheroma volume in African-American patients (+1.83±2.29 vs. –2.08±1.53 mm3, P=0.02). Caucasians demonstrated greater reduction in EEM volume (–13.64±2.58 vs. –5.79±4.03 mm3, P=0.01). However, this comparison was not statistically significant in the propensity matched patients (–13.41±3.43 vs. –7.59±4.61 mm3, P=0.18). There were no significant differences in change in percent atheroma volume and lumen volume between two groups.

Full Table
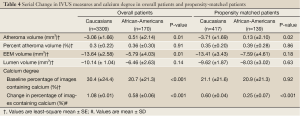
Full Table
At baseline, despite a similar overall plaque burden, African-American patients demonstrated a lower percentage of images containing calcium (20.7% vs. 30.4%, P<0.001) (Table 4). In contrast to greater disease progression, a smaller increase in percentage of images containing calcium was observed in African-American patients (+0.58%±0.06% vs. +1.08%±0.01%, P<0.001), a finding that persisted upon propensity matching (+0.25%±0.07% vs. +0.60%±0.04%, P<0.001).
Discussion
In addition to the ability to evaluate the impact of medical therapies, serial imaging with IVUS permits the ability to characterize the natural history of disease progression in different groups of patients (12-20). In the current analysis, African-American patients with CAD demonstrated a greater prevalence and less optimal control of coronary risk factors. In parallel, greater progression of coronary atherosclerosis was observed in African-American patients, a finding that persisted after propensity matching for differences in risk factors. These findings suggest a more aggressive form of disease that requires intensification of secondary prevention strategies in African-Americans.
The current study illustrated suboptimal risk factor control in the African-American patients. This was found, despite a relatively high rate of use of established therapies. This contrasts with typical reports in clinical practice that African-Americans have reduced access to care and use of established therapies (6-8). Moreover, African-Americans are under-represented in randomized clinical trials, including the current analysis where they contributed only five percent of the entire cohort. The observed differences in the current pooled analysis, despite relatively small numbers of African-American patients, further emphasizes the differences in risk factor control and plaque progression demonstrated between the groups.
The finding that on-treatment blood pressure remained higher, even after propensity matching, highlights its importance in African-American patients. This is reflected by the high rate of hypertensive complications, including stroke, left ventricular hypertrophy, renal dysfunction and heart failure, in the African-American community (21-23). Previous interventional studies have reported the limited blood pressure reductions with angiotensin converting enzyme inhibitors and angiotensin receptor blockers in African-Americans (24-26). Considering that the presence of polymorphisms of the promoter regions of aldosterone synthase and angiotensin II type receptor genes may increase the concentration of angiotensin converting enzyme and stimulate aldosterone synthesis (27), these backgrounds may contribute to promoting elevations in blood pressure and hyporesponsiveness to antihypertensive therapy. Accordingly, given that blood pressure control is associated with progression of coronary atherosclerosis (28), a greater need to achieve adequate blood pressure control using optimal anti-hypertensive agents and doses are required.
Despite higher level of HDL-C in African-Americans, greater progression of atheroma burden was observed. This lipid profile is consistent with previous studies (29,30), suggesting that HDL-C might not work as atheroprotective in African-Americans. Population studies have consistently observed an inverse relationship between systemic levels of HDL-C and cardiovascular risks (31). However, high levels of HDL-C are not always protective in subjects. In addition, findings from recent studies support the potential importance of HDL functionality in atherosclerosis rather than quality of HDL (32,33). These observations may indicate dysfunctional property of HDL in African-Americans, potentially contributing to progressive disease substrate. Further investigation will be required to elucidate quality of HDL in this minority.
Greater progression of coronary atherosclerosis persisted in African-American patients, after controlling for major risk factors. This suggests the presence of an aggressive form of disease, which may be promoted, to some degree, by events beyond established risk factors. However, there has been limited investigation into potential differences in atherogenic mechanisms between African-American and Caucasian subjects. While observations of a greater prevalence of endothelial dysfunction in African-American individuals are consistent with reports of reduced nitric oxide bioavailability (9-11), to what degree these abnormalities contribute to more aggressive forms of atherosclerosis is unknown. Findings that myeloperoxidase levels associate with atherosclerotic burden only in African-Americans may reflect a greater contribution of heightened inflammatory and oxidative pathways in disease progression (34). Further mechanistic investigation would be required to elucidate atherogenic pathway implicated in natural history of coronary atherosclerosis in African-American patients.
Recently, genome wide association studies have identified a region near the PFTK1 gene associated with incident cardiovascular events and subclinical atherosclerosis in African-Americans (35). This gene encodes a serine/threonine-protein kinase which regulates cell cycle progression and proliferation, suggesting potential alternative mechanisms contributing to greater progression of coronary atherosclerosis in African-Americans.
While atheroma volume significantly regressed in Caucasians, there were no significant differences in change in percent atheroma volume between two groups. As Caucasians tended to exhibit the vessel shrinkage reflected by more reduction in EEM volume, this vascular dimentional change would contribute to the same degree of change in PAV between two groups.
We also observed less plaque calcification, both at baseline and progression on serial evaluation, supporting reports from pathology studies (36,37). While racial differences in bone metabolism such as serum level of 1α,25(OH)2D3 have been reported (38,39), it remains to be fully elucidated whether this contributes to differences in plaque calcification. Atheroma burden with less plaque calcification may reflect a more vulnerable form of atherosclerosis, which potentially leads to the greater prevalence of future cardiovascular events in African-American patients with CAD.
A number of caveats should be noted with regard to these observations. The analysis pooled data from 7 clinical trials (14-20), which investigated the potential efficacy of anti-atherosclerotic agents, none of which randomized patients on the basis of race or ethnicity. While there may be heterogeneity of clinical characteristics between trials, adjustment for these differences revealed persistent greater disease progression in African-American patients. However, the possibility of residual confounding cannot be excluded. All patients underwent a clinically indicated coronary angiogram. As a result, it is unknown if the current findings can be extrapolated to asymptomatic individuals and the setting of primary prevention. It is not known whether progression rates are greater in patients, in which access to medical therapies and subsequent ability to control risk factors, is limited. Conventional ultrasonographic imaging provides a suboptimal characterization of plaque composition, precluding assessment of differences in these parameters between the groups. While previous studies have reported an association between disease progression and clinical outcome (40), the ultimate translation of these findings to differences in cardiovascular events remains to be determined.
African-American patients demonstrated less optimal control of risk factors and greater progression of coronary atherosclerosis, which persists after controlling for differences in clinical characteristics. These findings highlight an aggressive form of atherosclerosis and the need for more intensive efforts to target major cardiovascular risk factors in African-Americans.
Acknowledgements
We thank the technical expertise of the Intravascular Core Laboratory of the Cleveland Clinic.
Disclosure: Steven E. Nissen has received research support to perform clinical trials through the Cleveland Clinic Coordinating Center for Clinical Research from Pfizer, AstraZeneca, Novartis, Roche, Daiichi-Sankyo, Takeda, Sanofi-Aventis, Resverlogix, and Eli Lilly; and is a consultant/advisor for many pharmaceutical companies but requires them to donate all honoraria or consulting fees directly to charity so that he receives neither income nor a tax deduction.
Stephen J Nicholls has received speaking honoraria from AstraZeneca, Pfizer, Merck Schering-Plough and Takeda, consulting fees from AstraZeneca, Pfizer, Merck Schering-Plough, Takeda, Roche, NovoNordisk, LipoScience and Anthera and research support from AstraZeneca and Lipid Sciences.
All other authors have reported that they have no relationships to disclose.
References
- Yancy CW, Benjamin EJ, Fabunmi RP, et al. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: executive summary. Circulation 2005;111:1339-49. [PubMed]
- Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006;113:e85-151. [PubMed]
- Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 1995;25:305-13. [PubMed]
- Haffner SM, D’Agostino R Jr, Goff D, et al. LDL size in African Americans, Hispanics, and non-Hispanic whites: the insulin resistance atherosclerosis study. Arterioscler Thromb Vasc Biol 1999;19:2234-40. [PubMed]
- Hutchinson RG, Watson RL, Davis CE, et al. Racial differences in risk factors for atherosclerosis. The ARIC Study. Atherosclerosis Risk in Communities. Angiology 1997;48:279-90. [PubMed]
- Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites. N Engl J Med 2004;351:575-84. [PubMed]
- Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA 1999;282:583-9. [PubMed]
- LaVeist TA, Arthur M, Morgan A, et al. The cardiac access longitudinal study. A study of access to invasive cardiology among African American and white patients. J Am Coll Cardiol 2003;41:1159-66. [PubMed]
- Campia U, Choucair WK, Bryant MB, et al. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol 2002;40:754-60. [PubMed]
- Perregaux D, Chaudhuri A, Rao S, et al. Brachial vascular reactivity in blacks. Hypertension 2000;36:866-71. [PubMed]
- Mulukutla SR, Venkitachalam L, Bambs C, et al. Black race is associated with digital artery endothelial dysfunction: results from the Heart SCORE study. Eur Heart J 2010;31:2808-15. [PubMed]
- von Birgelen C, Hartmann M, Mintz GS, et al. Relationship between cardiovascular risk as predicted by established risk scores versus plaque progression as measured by serial intravascular ultrasound in left main coronary arteries. Circulation 2004;110:1579-85. [PubMed]
- Nicholls SJ, Tuzcu EM, Sipahi I, et al. Intravascular ultrasound in cardiovascular medicine. Circulation 2006;114:e55-9. [PubMed]
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004;291:1071-80. [PubMed]
- Nissen SE, Tuzcu EM, Libby P, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA 2004;292:2217-25. [PubMed]
- Nissen SE, Tuzcu EM, Brewer HB, et al. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 2006;354:1253-63. [PubMed]
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006;295:1556-65. [PubMed]
- Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med 2007;356:1304-16. [PubMed]
- Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 2008;299:1561-73. [PubMed]
- Nissen SE, Nicholls SJ, Wolski K, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA 2008;299:1547-60. [PubMed]
- Ruland S, Gorelick PB. Stroke in Black Americans. Curr Cardiol Rep 2005;7:29-33. [PubMed]
- Martins D, Tareen N, Norris KC. The epidemiology of end-stage renal disease among African Americans. Am J Med Sci 2002;323:65-71. [PubMed]
- Afzal A, Ananthasubramaniam K, Sharma N, et al. Racial differences in patients with heart failure. Clin Cardiol 1999;22:791-4. [PubMed]
- Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 1993;328:914-21. [PubMed]
- Weir MR, Gray JM, Paster R, et al. Differing mechanisms of action of angiotensin-converting enzyme inhibition in black and white hypertensive patients. The Trandolapril Multicenter Study Group. Hypertension 1995;26:124-30. [PubMed]
- Mallion JM, Goldberg AI. Global efficacy and tolerability of losartan, an angiotensin II subtype 1-receptor antagonist, in the treatment of hypertension. Blood Press Suppl 1996;2:82-6. [PubMed]
- Henderson SO, Haiman CA, Mack W. Multiple Polymorphisms in the renin- angiotensin-aldosterone system (ACE, CYP11B2, AGTR1) and their contribution to hypertension in African Americans and Latinos in the multiethnic cohort. Am J Med Sci 2004;328:266-73. [PubMed]
- Mulukutla SR, Venkitachalam L, Bambs C, et al. Black race is associated with digital artery endothelial dysfunction: results from the Heart SCORE study. Eur Heart J 2010;31:2808-15. [PubMed]
- Harris-Hooker S, Sanford GL. Lipids, lipoproteins and coronary heart disease in minority populations. Atherosclerosis 1994;108:S83-104. [PubMed]
- Haffner SM, D’Agostino R Jr, Goff D, et al. LDL size in African Americans, Hispanics, and non-Hispanic whites: the insulin resistance atherosclerosis study. Arterioscler Thromb Vasc Biol 1999;19:2234-40. [PubMed]
- Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989;79:8-15. [PubMed]
- Bhattacharyya T, Nicholls SJ, Topol EJ, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008;299:1265-76. [PubMed]
- Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127-35. [PubMed]
- Chen LQ, Rohatgi A, Ayers CR, et al. Race-specific associations of myeloperoxidase with atherosclerosis in a population-based sample: the Dallas Heart Study. Atherosclerosis 2011;219:833-8. [PubMed]
- Barbalic M, Reiner AP, Wu C, et al. Genome-wide association analysis of incident coronary heart disease (CHD) in African Americans: a short report. PLoS Genet 2011;7:e1002199. [PubMed]
- Eggen DA, Strong JP, McGill HC Jr. Coronary calcification. Relationship to clinically significant coronary lesions and race, sex, and topographic distribution. Circulation 1965;32:948-55. [PubMed]
- Strong JP, Mcgill HC Jr. The natural history of aortic atherosclerosis: relationship to race, sex, and coronary lesions in new orleans. Exp Mol Pathol 1963;52:15-27. [PubMed]
- Doherty TM, Tang W, Dascalos S, et al. Ethnic origin and serum levels of 1alpha,25-dihydroxyvitamin D3 are independent predictors of coronary calcium mass measured by electron-beam computed tomography. Circulation 1997;96:1477-81. [PubMed]
- Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation 1997;96:1755-60. [PubMed]
- Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399-407. [PubMed]


