
Combination of HGF and IGF-1 promotes connexin 43 expression and improves ventricular arrhythmia after myocardial infarction through activating the MAPK/ERK and MAPK/p38 signaling pathways in a rat model
Introduction
According to current epidemiological data, cardiovascular diseases now account for approximately one third of all deaths globally (1). Sudden cardiac death (SCD), which is mainly caused by ventricular tachyarrhythmia, is the leading cause of death in myocardial infarction (MI) patients. Beneficial effects on infarction size, systolic function and ventricular remodeling have been found with factor-based therapy in MI (2,3). The combination of insulin-like growth factor-1 (IGF-1) and hepatocyte growth factor (HGF), mostly as an adjunct to stem cell therapy, has already been well studied and has been shown to have considerable efficacy in myocardial repair (4,5). However, the efficacy of cell-based regenerative therapies is still debated, and 5-year follow-up data in randomized clinical trials have shown no improvement in overall survival (6).
Since the antiarrhythmic effect of growth factors (GFs) used independently and the relationship between these GFs have been barely explored and the underlying mechanisms remain elusive, we detected changes in the expression of connexin 43 (Cx43), a major gap junction protein that is closely associated with the occurrence of fatal arrhythmias (7-10), and the electrophysiological consequences after the intramyocardial administration of GFs in a rat model. MAPK/ERK and MAPK/p38 signaling pathways, which may be involved in this process, were investigated by using corresponding inhibitors to explore the underlying mechanisms.
Methods
NRVM isolation and culture
One- to 2-day-old Sprague-Dawley rats were used to isolate neonatal rat ventricular myocytes (NRVMs), and the NRVMs were cultured in vitro. Rat hearts were excised, and the atrium, blood clots and fibrous tissue around the heart were removed. Then, the heart was minced into 1-mm3 pieces in an aseptic culture dish with 4 °C phosphate buffer solution (PBS) under sterile conditions. The pieces were then washed three times with PBS to remove impurities. Then, 2–3 mL of 0.2% trypsin was added for digestion, the dishes were gently shaken for 7 to 10 mins in a 37 °C water bath, the tissues were precipitated and the first supernatant was discarded. Digestion/precipitation steps were performed an additional 7–10 times until the tissue was digested completely. The collected supernatants were then added to complete explant medium (CEM) supplemented with 10% fetal calf serum, filtered through a cell strainer (BD Biosciences, USA), and centrifuged at 2,500 r/min for 135 s at 4 °C. The cells were then resuspended, and cardiomyocytes were separated by differential adhesion. Nonadherent cells were removed, plated in 6-well plates at a density of 2.0×105 cells/well and incubated at 37 °C in culture medium. The medium was changed two days after isolation. Cell beating was observed in most NRVMs after 72 hours.
Grouping of NRVMs
NRVMs were divided into 8 groups 96 hours after isolation and treated with PBS, HGF (50 ng/mL), IGF-1 (100 ng/mL), GFs (50 ng/mL HGF + 100 ng/mL IGF-1), HGF + p38 inhibitor (SB203580, 50 µM), HGF + ERK inhibitor (PD98059, 100 µM), IGF-1 + p38 inhibitor, or IGF-1 +ERK inhibitor in culture medium for 48 hours at the indicated concentrations. The growth factors were dissolved in PBS, and the inhibitors were dissolved in DMSO according to the manufacturer’s instructions. Each group of NRVMs was pretreated with DMSO.
Western blot analysis
Protein was extracted from the cells and tissues using RIPA buffer. A Bradford assay was used to measure the protein concentration. Equal amounts of protein were loaded and separated by SDS-PAGE and then transferred onto polyvinylidene fluoride membranes (Millipore, Boston, USA). The membranes were incubated with anti-Cx43 (ab11370, polyclonal antibody; dilution 1:1,000; Abcam, USA) and anti-GAPDH (1:2,000; Abcam, USA) primary antibodies overnight at 4 °C. The antigen-antibody reaction was visualized by enhanced chemiluminescence after washing. Band intensity was analyzed by imaging software (ImageJ 2X, version 2.1.4.7), and the experiments were performed in triplicate.
Reverse transcription quantitative polymerase chain reaction (RTqPCR)
Total RNA was isolated with TRIzol reagent (Invitrogen, CA, USA) and then reverse transcribed into cDNA. Real-time PCR was performed to detect the expression of related genes. The primers used are listed in Table 1. The threshold cycle of the target genes was normalized to that of β-actin, relative mRNA expression was quantified using the 2−ΔΔCt method, and the experiments were performed in triplicate.
Full table
Preparation of rat MI model
Forty-eight adult male Sprague-Dawley (SD) rats weighing 300–350 g were anesthetized with 1% pentobarbital sodium (50 mg/kg) by intraperitoneal injection. A standard limb lead II electrocardiogram (ECG) was continuously recorded with an ECG recorder (BIOPAC MP150). Tracheal cannulation via the mouth was performed, and the animals were mechanically ventilated with room air at a tidal volume of 0.5 mL/100 g of body weight, a breathing ratio of 2:1 and a frequency of 100 breaths/min. The heart was exposed using an angular incision on the left side of the thoracic cavity, the pericardium was incised and the left anterior descending coronary artery was exposed. The left anterior descending coronary artery was ligated 2 mm below the left atrial appendage by using a 5/0 nylon suture. The residual blood and gas were sucked out from the thoracic drainage tube before the chest was closed. Successful occlusion was confirmed by the elevation of the ST segment by electrocardiograph and regional cyanosis of the myocardial surface. When the rats awoke and exhibited swallowing, the tracheal intubation was pulled out. An injection of 400,000 U of penicillin after the operation was administered to prevent infection. All animal handling and procedures were performed in accordance with protocols that were approved by the Animal Ethics Committee of Sun Yat-sen University.
Grouping of MI rats
Two weeks after the operation, the 34 surviving rats were randomly divided into 4 groups: 8 in the PBS group, 9 in the HGF group, 9 in the IGF-1 group, and 8 in the GFs group. The rats were anesthetized, mechanically ventilated as described above, and then PBS, 10 ng HGF, 20 ng IGF-1 or GFs (10 ng HGF + 20 ng IGF-1) in a total volume of 400 µL were locally injected into the infarcted border area. The rats were allowed to recover after injection.
Induction of ventricular arrhythmias
Six weeks after factor-based treatment, ventricular arrhythmias (VAs) were induced in the rats by programmed stimulation. The rats were anesthetized, and the heart was exposed as described above. Electrocardiograms were recorded continuously with a limb lead. Bipolar pacemaker electrodes were placed on the apex of the left ventricle, and a DF-6A programmed electric stimulator was used to perform S1S2, S1S2S3 and burst stimulation. The duration of one cycle of S1S2, including 8 base stimuli and 1 additional stimulus, was 120 ms. The duration of one cycle of S1S2S3, including 8 base stimuli and 2 additional stimuli, was 120 ms. For burst stimulation, 100/90/80/70/60/50-ms stimuli over a period of 1 s were applied. The induction of sustained ventricular tachycardia (VT) and/or ventricular fibrillation (VF) by any type of stimulation was considered to be the successful induction of VAs. A VT duration of >3 s was defined as sustained ventricular tachycardia.
Immunohistochemical staining
The localization and distribution of Cx43 in the infarct border zone of the left ventricle was investigated by immunohistochemical staining. The border zone of the left ventricular heart tissue 2 mm from the infarct edge was collected, embedded in paraffin and serially sectioned (5 µm thick). The slides were deparaffinized with xylene and rehydrated with ethanol, and antigen retrieval was performed in 0.01 M citrate buffer (pH 6.0) in a pressure cooker, followed by natural cooling to room temperature. Then, incubation in 0.3% hydrogen peroxide at RT for 10 min was performed. Goat serum was used to block the sections for 15 min at RT. The slices were incubated with a rabbit polyclonal anti-Cx43 antibody (1:1,000 dilution; Abcam, UK) overnight at 4 °C and then incubated with an HRP-conjugated goat anti-rabbit IgG antibody (ABclonal, Wuhan, China) for 30 min at RT. Immunodetection was performed using DAB solution according to the manufacturer’s instructions. Following washing, the slides were counterstained, dehydrated, and then coverslipped using neutral gum sealant.
Measurement of Cx43 levels in the infarct border zone
The mRNA and protein expression of Cx43 in the infarct border zone was detected via qRT-PCR and Western blotting eight weeks after infarction. The primers used for qRT-PCR are shown in Table 2, and the experiments were performed in triplicate.
Full table
Statistical analysis
SPSS 20.0 statistical software was used for statistical analysis. To ensure the accuracy of data in general, all experiments were performed in triplicate and the quantitative data were recorded as the mean ± SD. The normality and variance homogeneity test was performed before statistical analysis. Single-factor variance analysis was used for multiple groups. The chi-square test and Fisher’s exact test were used for qualitative data. The LSD and Bonferroni tests were used for pairwise comparison. A value of P<0.05 was considered statistically significant.
Results
Effect of GFs on Cx43 expression in NRVMs
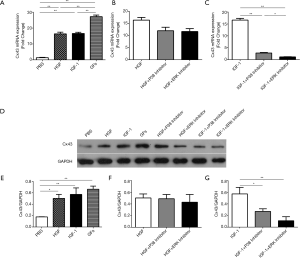
The expression of Cx43 mRNA in NRVMs was higher in the HGF, IGF-1, and GFs group than in the PBS group (Figure 1A, P<0.01, P<0.01, P<0.01, respectively). And combinatorial effect was observed in GFs group compared to HGF and IGF-1 group (Figure 1A, P<0.01, P<0.01, respectively). No significant differences in the Cx43 mRNA levels were observed between the HGF + p38 inhibitor group and the HGF + ERK inhibitor group (Figure 1B, P>0.1). There was a significant reduction in the Cx43 mRNA levels in the IGF-1 + p38 inhibitor group and IGF-1 + ERK inhibitor group compared to the IGF-1 group (Figure 1C, P<0.01, P<0.01, respectively). Western blot analysis reveals similar expression pattern but combinatorial effect was not found in GFs groups (Figure 1D,E,F,G).
GFs reduced the frequency of induced ventricular arrhythmia in MI rats
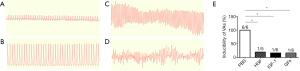
A total of 48 rats were used in the experiment. The survival rates were 70.83% (34/48) after MI and 47.92% (23/48) after reoperation, and no significant difference was observed between the groups. Successful model establishment was confirmed by the elevation of the ST segment by electrocardiograph (Figure 2A,B) and the regional cyanosis of the myocardial surface. Ventricular arrhythmias (VAs) (Figure 2C,D) were induced in all 6 rats in the PBS group, in 1 of the 5 rats in the HGF group, in 1 of the 6 rats in the IGF-1 group, and in 1 of the 6 rats in the GFs group. The HGF, IGF-1, and GFs groups exhibited lower inducibility of VAs than that in the PBS group (Figure 2E, P<0.05, P<0.05, P<0.05, respectively), there was no significant difference between the HGF, IGF-1, and GFs groups.
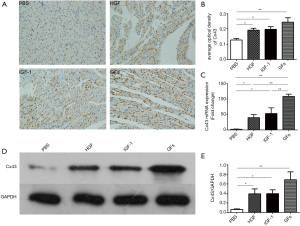
Effect of GFs on Cx43 expression in the infarct border zone
The expression of Cx43 in the infarct border zone of the left ventricle, as detected by immunohistochemical staining, was higher in the HGF, IGF-1, and GFs groups than in the PBS group (Figure 3A,B, P<0.05, P<0.05, P<0.01). The Cx43 mRNA and protein levels in the cardiac muscular tissue were determined. Both the mRNA and protein levels of Cx43 in the infarct border zone of the left ventricle were higher in the HGF, IGF-1, and GFs groups than in the PBS group (Figure 3C,D,E, P<0.05, P<0.05, P<0.01). GFs have complementary effects on Cx43 mRNA expression but not on Cx43 protein levels in the infarct border zone of the left ventricle, as determined by immunohistochemistry and Western blotting.
Discussion
MI is a severe type of coronary heart disease with high mortality. MI can have dire consequences, and patients can die of SCD caused by persistent VT/VF. Cx43 reconstruction is one of the structural changes after MI that causes arrhythmias (9,11,12). The abnormal expression and distribution of Cx43 may lead to a decrease in intercellular conduction and coupling ability, resulting in a variety of reentrant arrhythmias (13). Cx43 expression is significantly reduced or eliminated in the infarct zone and border zone after acute focal ischemia (14,15), and this effect is accompanied by Cx43 redistribution, which undermines the conduction velocity and the inhomogeneous anisotropy of cardiac conduction, causing severe ventricular arrhythmias (16,17). Cx43 remodeling plays a significant role in the pathogenesis and prognosis of ischemic arrhythmias, and interventions targeting Cx43 remodeling may reduce the occurrence of ventricular arrhythmias after MI. In our study, Cx43 expression was significantly reduced in the infarct zone and border zone of rats six weeks after surgical intervention.
Various factors have been investigated for factor-based therapy for myocardial injury, with relatively more attention being paid to HGF and IGF-1. The HGF/Met pathway plays a significant role in cardiovascular repair after myocardial injury, including ischemic injury and cardiogenic toxicity caused by chemotherapy drugs (3). These stimuli activate the Met pathway, exert strong antiapoptotic effects on cardiomyocytes (18-20), and induce endothelial cell migration by activating Rac1 (21). A study on a pig model of MI showed that treatment with mesenchymal stem cells with modifications in the HGF gene (HGF-MSCs) increases vascular density and Cx43 expression and induces a lower apoptosis rate and a reduced occurrence of ventricular arrhythmias compared with those induced by treatment with unmodified MSCs (22). Intriguingly, the presence of HGF alone recapitulates most of the effects shown in our research. Another factor, IGF-1, however, functioning in a distinct way, inhibits hypoxia-induced cardiomyocyte apoptosis and protects cardiomyocytes from reperfusion injury by activating MAPK, PI3K and Raf-1 in the myocardium; this is congruent with our study, as demonstrated by the use of p38 MAPK and ERK inhibitors. Studies have suggested that there is a causal relationship between IGF-1 levels and the incidence of cardiovascular disease, as a low concentration of IGF-1 is positively correlated with an increased risk of MI, angina and myocardial ischemia (23,24). Similarly, compared with untreated MSCS, MSCs pretreated with IGF-1 exhibit a significantly increased abundance of Cx43 in the myocardium and decreased levels of inflammatory factors after MI indicating the cardioprotective effect of IGF-1 (25).
In our study, the in vitro treatment of NRVMs with HGF and IGF-1 markedly upregulated Cx43 expression. The intramyocardial injection of both HGF and IGF-1 increased Cx43 mRNA and protein levels in cardiomyocytes in the infarct border zone in MI rats, and a lower incidence of ventricular arrhythmias was observed compared to that in PBS controls, confirming the involvement of these factors in Cx43 reconstruction and their beneficial role in improving the electrical coupling performance of the myocardium. Notably, we found a significant reduction in Cx43 levels after applying a p38 or ERK inhibitor in IGF-1-treated NRVMs, which indicates that IGF-1 may increase the expression of Cx43 through the MAPK/p38 and ERK1/2 signaling pathways, while some other dominant pathways involving HGF may be involved in this process. As shown above, we conclude that the combined administration of HGF and IGF-1 may be synergistic.
Studies have shown that the injection of human umbilical cord-derived mesenchymal stem cells significantly improves cardiac function by increasing the expression of HGF and IGF-1 in doxorubicin-induced cardiomyopathy, suggesting the importance of paracrine actions in cell-based therapy (26,27). In our findings, the intramyocardial administration of GFs in a rat model of MI reduced the occurrence of ventricular arrhythmias to a level that was comparable to that induced by IGF-1 or HGF alone, and no synergistic effect was found on Cx43 protein expression. Notably, the additive effect of GFs on Cx43 expression was only found at the mRNA level in vitro, as shown by our data. Since only a single concentration and one time point were used in our study, further optimizations are needed concerning the timing of treatment, the adequate ratio, and the frequency of injection. Regarding the unparalleled change between the mRNA and protein levels of Cx43, multiple underlying mechanisms, including various post transcriptional modifications induced by GFs treatment, may be involved.
Conclusions
In summary, we investigated the regulatory role of HGF and IGF-1 on Cx43 expression and the resulting antiarrhythmic function, and preliminary explorations on the underlying pathways that may be involved, which may exhibit therapeutic potential for ventricular arrhythmias after MI, were also carried out. Given that factor-based therapy is a promising and rather simplified strategy compared with the many challenging cell-based therapies for MI treatment, the cardioprotective effects of HGF and IGF-1 alone and in combination, as well as the associated mechanisms, warrant more specific investigations in the context of MI.
Acknowledgments
Funding: This work was supported by the Science and Technology Planning Project of Guangdong Province (grant No. 2014A020212088), the Major Program of Science and Technology Planning Project in Medical and Health of Zhuhai (grant No. 2015B1031) and the Phoenix Project of the Fifth Affiliated Hospital of Sun Yat-Sen University (J Ke).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The animals in this study were obtained from the Animal Experimental Center of Sun Yat-sen University. All animal handling and procedures were performed in accordance with protocols that were approved by the Animal Ethics Committee of Sun Yat-sen University. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Joseph P, Leong D, McKee M, et al. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ Res 2017;121:677-94. [Crossref] [PubMed]
- Xue J, Du G, Shi J, et al. Combined treatment with erythropoietin and granulocyte colony-stimulating factor enhances neovascularization and improves cardiac function after myocardial infarction. Chin Med J (Engl) 2014;127:1677-83. [PubMed]
- Awada HK, Long DW, Wang Z, et al. A single injection of protein-loaded coacervate-gel significantly improves cardiac function post infarction. Biomaterials 2017;125:65-80. [Crossref] [PubMed]
- Ruvinov E, Leor J, Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials 2011;32:565-78. [Crossref] [PubMed]
- Hwang H, Kloner RA. Improving regenerating potential of the heart after myocardial infarction: factor-based approach. Life Sci 2010;86:461-72. [Crossref] [PubMed]
- Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med 2006;355:1222-32. [Crossref] [PubMed]
- Lübkemeier I, Requardt RP, Lin X, et al. Deletion of the last five C-terminal amino acid residues of connexin43 leads to lethal ventricular arrhythmias in mice without affecting coupling via gap junction channels. Basic Res Cardiol 2013;108:348. [Crossref] [PubMed]
- Cutler MJ, Jeyaraj D, Rosenbaum DS. Cardiac electrical remodeling in health and disease. Trends Pharmacol Sci 2011;32:174-80. [Crossref] [PubMed]
- Fontes MS, van Veen TA, de Bakker JM, et al. Functional consequences of abnormal Cx43 expression in the heart. Biochim Biophys Acta 2012;1818:2020-9. [Crossref] [PubMed]
- Severs NJ, Bruce AF, Dupont E, et al. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res 2008;80:9-19. [Crossref] [PubMed]
- Martins-Marques T, Catarino S, Marques C, et al. To beat or not to beat: degradation of Cx43 imposes the heart rhythm. Biochem Soc Trans 2015;43:476-81. [Crossref] [PubMed]
- Martins-Marques T, Catarino S, Zuzarte M, et al. Ischaemia-induced autophagy leads to degradation of gap junction protein connexin43 in cardiomyocytes. Biochem J 2015;467:231-45. [Crossref] [PubMed]
- Beardslee MA, Lerner DL, Tadros PN, et al. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res 2000;87:656-62. [Crossref] [PubMed]
- Schulz R, Gorge PM, Gorbe A, et al. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol Ther 2015;153:90-106. [Crossref] [PubMed]
- Zhai H, Dai W, Wang Y. Metoprolol protects cardiomyocytes in rabbit model of heart failure by regulating Cx43. Exp Ther Med 2018;15:1902-5. [PubMed]
- Ciaccio EJ, Ashikaga H, Coromilas J, et al. Model of bipolar electrogram fractionation and conduction block associated with activation wavefront direction at infarct border zone lateral isthmus boundaries. Circ Arrhythm Electrophysiol 2014;7:152-63. [Crossref] [PubMed]
- Savi M, Bocchi L, Rossi S, et al. Antiarrhythmic effect of growth factor-supplemented cardiac progenitor cells in chronic infarcted heart. Am J Physiol Heart Circ Physiol 2016;310:H1622-48. [Crossref] [PubMed]
- Zhou XL, Wan L, Liu JC. Activated Notch1 reduces myocardial ischemia reperfusion injury in vitro during ischemic postconditioning by crosstalk with the RISK signaling pathway. Chin Med J (Engl) 2013;126:4545-51. [PubMed]
- Yi X, Li X, Zhou Y, et al. Hepatocyte growth factor regulates the TGF-beta1-induced proliferation, differentiation and secretory function of cardiac fibroblasts. Int J Mol Med 2014;34:381-90. [Crossref] [PubMed]
- Gallo S, Gatti S, Sala V, et al. Agonist antibodies activating the Met receptor protect cardiomyoblasts from cobalt chloride-induced apoptosis and autophagy. Cell Death Dis 2014;5:e1185. [Crossref] [PubMed]
- Gallo S, Sala V, Gatti S, et al. Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clin Sci (Lond) 2015;129:1173-93. [Crossref] [PubMed]
- Zhang J, Wang LL, Du W, et al. Hepatocyte growth factor modification enhances the anti-arrhythmic properties of human bone marrow-derived mesenchymal stem cells. PLoS One 2014;9:e111246. [Crossref] [PubMed]
- Saetrum Opgaard O, Wang PH. IGF-I is a matter of heart. Growth Horm IGF Res 2005;15:89-94. [Crossref] [PubMed]
- Spallarossa P, Brunelli C, Minuto F, et al. Insulin-like growth factor-I and angiographically documented coronary artery disease. Am J Cardiol 1996;77:200-2. [Crossref] [PubMed]
- Guo Jun LZ. Effects of insulin-like growth factor 1 on the myocardium regeneration and anti-inflammation in mesenchymal stem cells Journal of Clinical Cardiology 2011:948-51.
- Singla DK, Ahmed A, Singla R, et al. Embryonic stem cells improve cardiac function in Doxorubicin-induced cardiomyopathy mediated through multiple mechanisms. Cell Transplant 2012;21:1919-30. [Crossref] [PubMed]
- Mao C, Hou X, Wang B, et al. Intramuscular injection of human umbilical cord-derived mesenchymal stem cells improves cardiac function in dilated cardiomyopathy rats. Stem Cell Res Ther 2017;8:18. [Crossref] [PubMed]


