
Non-invasive assessment of liver alterations in Senning and Mustard patients
Introduction
The atrial switch operation (TGA-ASO; Senning or Mustard procedure) was the initial surgical treatment option for subjects with transposition of the great arteries, and the medical standard from the mid 1960s to the mid 1980s (1,2). Although the surgical technique has nowadays been replaced by the arterial switch technique, survivors of TGA-ASO still make a relevant part of the adult congenital heart disease (ACHD) population (3).
Current publications about the long-term outcome from TGA-ASO identify rhythm problems as supraventricular tachycardia, ventricular arrhythmias as well as heart failure and the need for surgical re-interventions as main problems during the late follow-up (3-5).
Non-cardiac complications significantly contribute to the morbidity and mortality of ACHD leading to an epidemiological shift and a generation of patients who are at risk of developing chronic multisystem disease in adulthood (3,6). Liver dysfunction is one of the non-cardiac complications of ACHD that requires surveillance according to the current guidelines, which includes the recommendations for liver surveillance in ACHD (3,6-8). Research in this field was mainly done on Fontan-associated liver disease. Several studies demonstrated consistently near-universal fibrosis both early and late after Fontan procedures (3,9-12). Studies based on non-invasive imaging detected in patients with Fontan physiology 57–67% ultrasound abnormalities of the liver, and in 72–100% liver abnormalities using computed tomography or magnetic resonance imaging (3,13-16). Data are limited in other ACHD (3,17,18).
The purpose of the present study was to examine hepatic abnormalities of adults after atrial switch operation prospectively with non-invasive methods. Furthermore, a differentiated analysis between Senning and Mustard patients is attempted, hence the Senning operations were performed with autologous atrial septum tissue while the Mustard procedures relied on a patch from pericardium or—in the majority of cases—an artificial patch (Dacron®) with a possible impact on atrial compliance.
Methods
Adult patients with TGA-ASO were recruited in two tertiary care centres for ACHD. Patients were enrolled consecutively at the two institutions (German Heart Centre Munich, German Heart Centre Berlin, Germany) in an order that they showed up at the institutions, not selected by the physician in any particular way. Exclusion criteria were chronic infection with hepatitis B or C, missing cognitive skills, pregnancy, refusal of informed consent or technical obstacles for non-invasive liver stiffness measurements (e.g., obesity, narrow intercostal spaces or scares in the position range of the probe).
The study was in accordance with the principles outlined in the Declaration of Helsinki 1975 and was approved by the Institutional Review Board of the Technical University of Munich and the Charité University Berlin. Written informed consent was obtained from all participants.
All patients underwent investigations according to a prospective study based protocol which was selected after discussion with the hepatologists: clinical assessment, echocardiography, specific laboratory tests, abdominal sonography and ultrasound based liver stiffness measurements [transient elastography (TE) and acoustic radiation force impulse imaging (ARFI)]. Clinical assessment included a 6-minute walk test and NYHA functional classes. NYHA functional classes enable to assess the impact of heart failure: class 1: asymptomatic at all levels of activity; class 2: mild symptoms and light limitation during ordinary activity; class 3: marked limitation in activity due to symptoms, even during less-than-ordinary activity; class 4: severe limitations, advanced symptoms significantly curtail virtually all average everyday activity and may be present at rest. Time frame of patient enrollment and evaluation were 26 months starting from March 2010 till April 2012. All tests were performed in the same “time-period”. Due to organisational reasons and the multicenter aspect of the study, data of each patient were collected within a short time frame (days to some weeks).
Laboratory tests
Venous blood samples were examined using standard laboratory methods nearly followed the current guidelines for consideration for liver surveillance in adults with CHD: hemoglobin, hematocrit, erythrocytes, platelets, leukocytes, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, gamma-glutamyl transpeptidase (GGT), total bilirubin, cholinesterase (CHE), albumin, immunoglobulines, lipometabolism and iron metabolism parameters, kidney and thyroid function parameters, activated partial thromboplastin time (aPTT), prothrombin time, international normalized ratio (INR), fibrinogen, NTproBNP and hepatitis serologic testings.
Specific hepatic laboratory diagnostic included: AST to platelet ratio (APRI) index, fibrosis-4 (FIB-4) index, AST/ALT-ratio, nonalcoholic fatty liver disease (NAFLD) score, BARD score (composed of three variables: AST/ALT ratio, body mass index and the presence of diabetes) and FORNS score [composed of four variables: platelets (103/mm3), GGT (IU/L), age and cholesterol (mg/dL)] as indirect fibrotic markers (19). Hyaluronic acid concentrations were measured as direct fibrotic marker. Cut-off values for APRI index are ≥1.5 for fibrosis and ≥2 for cirrhosis, for FIB-4 index ≥1 for fibrosis and for hyaluronic acid ≥75 ng/mL for fibrosis (3,20-22). AST/ALT-ratio >1 was assumed to correlate with a severe hepatic disorder. In patients with an NAFLD fibrosis score below −1.455, advanced liver fibrosis can be excluded with high accuracy (23), while cut-off values for higher stages fibrosis for BARD and FORNS score are ≥2 and <4.2 respectively.
Abdominal sonography
Conventional abdominal ultrasound with high-resolution technique was performed by an experienced gastroenterologist looking for alterations of the hepatic parenchyma, diffuse focal hepatic or concomitant lesions, focusing on homogeneity of the hepatic tissue, liver echogenicity, presence of ascites, portal vein diameter, splenic vein diameter and spleen size as markers of portal hypertension (3,24).
Assessment of ventricular function
Echocardiography was reviewed by an experienced ACHD physician. Systemic right ventricular (RV) function was evaluated by qualitative assessment of clinical echocardiograms and classified as normal, mildly or severely depressed relying on “eye-balling” judgment. Tricuspid valve regurgitation (TR) was classified as absent, mild, moderate or severe. Special focus was on the detection of baffle stenosis and baffle leakage. Subpulmonary ventricular function (morphological left ventricle) was measured as ejection fraction from short axis view and mitral valve insufficiency was evaluated.
Non-invasive liver stiffness measurement
Liver stiffness measurements were obtained by two ultrasound based methods, performed by trained study investigators certified by the manufacturer and blinded to clinical data of the patients except for the diagnosis of the underlying congenital heart defect. Correct probe was used corresponding to the patient’s body type between M or XL followed by the automatic probe section tool.
For TE, the ultrasound transducer transmits a vibration of mild amplitude and low frequency, inducing an elastic shear wave that propagates through the liver and relates directly to the stiffness of the liver. On the basis of the propagation velocity of this shear wave, liver elasticity and density can be estimated. The faster the elastic shear wave propagates through the hepatic tissue, the more pronounced is the loss of elasticity.
Following a standard protocol, the measurements were repeated to 10 valid measurements, whereof the median is calculated, reflecting hepatic density. Results of the TE were expressed in kilopascal (kPa), ranging from 2.5–75 kPa and were correlated to the degree of liver stiffness. According to Foucher et al. (3,25), a cut-off value of <7.2 kPa was used for the classification of no or no mild fibrosis (F0–1), ≥7.2 kPa for moderate fibrosis (F2), ≥12.5 kPa for severe fibrosis (F3) and ≥17.6 kPa for liver cirrhosis (F4).
As second technique, ARFI (ACUSON S2000, Virtual Touch tissue; Siemens Healthcare, Erlangen) was applied for hepatic stiffness assessment at a frequency of 4 MHz. Thereby, hepatic tissue is deformed mechanically by a short-time acoustic impulse, generating shear waves perpendicular to the acoustic stimulus. The velocity of the shear wave propagation (in m/s) is detected by B-mode sonography and increases with the progression of hepatic stiffness. The median of 10 valid measurements was calculated and the results expressed as arithmetic average (3,26-28).
Fibrosis stages were set according to the recommendations of Fierbinteanu-Braticevici et al. (3,27): <1.185 m/s no fibrosis, ≥1.185 m/s mild, ≥1.215 m/s moderate, ≥1.540 m/s severe fibrosis and ≥1.940 m/s liver cirrhosis, corresponding to graduations of liver fibrosis 0–4.
Statistical analysis
Statistical analysis was performed using SPSS in its current version (version 24, SPSS Inc., Chicago, IL, USA). Qualitative variables were reflected as percentages of the total and continuous data are presented as median and range. For assessment of group differences concerning relevant parameters, Wilcoxon test for continuous variables was used. For all parameters, a value of P<0.05 was considered statistically significant.
Results
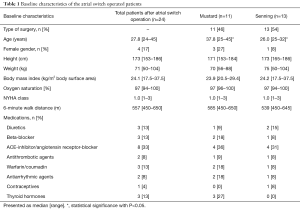
A total of 30 patients were enrolled. Four patients were excluded due to newly diagnosed hepatitis C (n=2) and hepatitis B (n=1), pregnancy (n=1) and non-attendance of two participants. The final study group comprised 24 patients with TGA-ASO (Senning n=13 and Mustard n=11). Five patients had a complex d-TGA with at least one significant additional CHD [ventricular septal defect (n=5), coarctation of the aorta (n=2), subpulmonary stenosis (n=3)]. Median age of the patients was 27.8 years (24–45 years) and the Mustard group was significantly older than the Senning group. Most of them (n=14; 58%) were within their third decade of life. Age at atrial switch operation ranged from 2 weeks to 23.9 years (median 0.9 years). Thirteen patients (54%) were operated within the first year of life. The interval between the last corrective cardiac operation and liver assessment was 13.9-38.3 years (median 26.4 years). None had a history of alcohol abuse.
Clinical assessment
At the time of study enrollment, all subjects were in stable clinical conditions without signs of acute cardiac decompensation or cutaneous signs of hepatic congestion and liver cirrhosis.
Fifteen patients (63%) were asymptomatic (NYHA class I), seven (29%) were in NYHA class II, and two patients in class III (8%). The 6 minute walk was available in 11 patients and ranged from 450 to 650 meters (median 557 m).
Oxygen saturation ranged from 94% to 100% (median 97%). Ten patients (42%) were on cardiac medication (diuretics, beta-blocker, ACE-inhibitors/angiotensin receptor-blocker or antiarrhythmic agents). Three patients (12.5%) had a pacemaker due to sick sinus syndrome, while all others presented with sinus rhythm. At the time of data collection, two patients with pacemaker were paced in the atrium (AAI), the third one presented with sinus rhythm. Baseline characteristics of the Senning and Mustard patients are provided in Table 1.
Full table
Echocardiography
Echocardiography provided “normal” systemic function of the morphologic right ventricle (RV) in 8/20 patients, mildly depressed RV function in 12/20 patients and none had a severely depressed RV function (missing data in four patients). Ventricular function did not correlate with hepatic alteration. No subject suffered from baffle stenosis or baffle leakage or had a mitral valve insufficiency. TR was measured in 20 patients: no (n=1), mild (n=12), moderate (n=7) and no severe TR. Median ejection fraction of the subpulmonary ventricle was 74% (54–88%).
Laboratory assessment
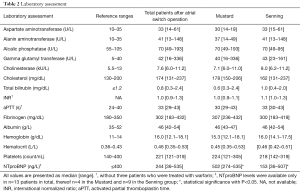
Two patients were treated with warfarin because of paroxysmal tachycardia and mildly reduced ventricular function and pacemaker. Their coagulation parameters were consequently excluded from the statistical analysis.
Fourteen of 24 patients (58%) presented with laboratory findings of hepatic disease or cholestasis. GGT was elevated in 10 patients (42%), while eight revealed an isolated GGT elevation and two had at least one additional pathologic liver parameter. One patient had elevated GGT in combination with elevated ALT, and one had a combination with elevated AP and total bilirubin. Total bilirubin was increased in 5/24 patients (21%), thereof in two cases with elevation of bilirubin and two additional pathologic liver parameters. None of the patients had substantially elevated transaminases.
Nine patients showed elevated NTproBNP values (median 244 ng/L, range 36–535 ng/L; normal <125 ng/L). NTpro BNP levels differed amongst the Mustard and Senning group, and were significantly higher in the Mustard group. Relevant laboratory parameters are shown in Table 2.
Full table
Serum markers of liver fibrosis
As specific serum marker of liver fibrosis, FIB-4 index, APRI index, AST/ALT-Ratio, NAFLD, BARD and FORNS score and hyaluronic acid were assessed. Valid results were available in all subjects for the FIB-4 and APRI index, AST/ALT-Ratio, NAFLD, BARD and FORNS score, but only in 19/24 subjects for hyaluronic acid. The hyaluronic acid concentration in samples from a normal population was <75 ng/mL (95th percentile).
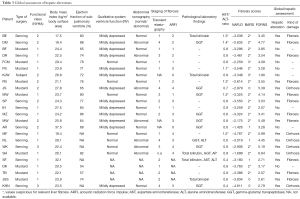
Based on an APRI index of ≥1.5 for liver fibrosis and ≥2 for liver cirrhosis, no patient had an abnormal APRI index >2. In addition, no patient had an abnormal FIB-4 index >1, none showed abnormally elevated APRI index or increased hyaluronic acid levels >75 ng/mL. AST/ALT-ratio >1 was found in six patients, whereof one had no fibrosis, four were classified to have fibrosis and one suffered from cirrhosis in the global hepatic assessment. NAFLD fibrosis score offered conspicuous values in only one patient, who was decided to have no relevant hepatic disorder in the global hepatic assessment, while the results of the BARD fibrosis score (see Table 3) were very unspecific and showed numerous false results compared to the global hepatic assessment. The FORNS score identified four patients correctly with cirrhosis, but even missed this to diagnose cirrhosis in two cases.
Full table
Abdominal sonography
Abdominal sonography was performed by an experienced hepatologist and was available in 20/24 patients while four examinations were not performed.
Examinations classified as abnormal were: enlarged hepatic veins (n=1), rounded liver edge (n=1), inhomogenious liver parenchyma (n=3) and presence of irregular liver surface (n=2). In total, 7/20 patients (35%) showed abdominal ultrasound abnormalities of the hepatic parenchyma and were classified by an experienced investigator as “not healthy liver”.
Liver stiffness measurements
All patients were subject to TE and/ or ARFI examination. In four patients of each method, measurements were technically not feasible. Technically infeasibility was due to abdominal gases (n=7) and obesity (n=1). Reliable results with TE and ARFI were obtained in each method with 83%. TE demonstrated mild fibrosis in eight patients, moderate fibrosis in two, severe fibrosis in three and cirrhosis in four patients. ARFI provided no fibrosis in three, mild fibrosis in four, moderate in four, severe in five and cirrhosis in four patients.
In summary, 16/24 patients (67%) had both, valid results in TE and ARFI examination.
Identical classification by both methods of hepatic staging was received in 4/16 patients (25%): three cases with mild fibrosis and one with liver cirrhosis. Similar results with only one degree difference in graduation was detected in 8/16 patients (50%). In three cases, TE and ARFI provided discrepant results: in two patients classified as liver fibrosis by global hepatic assessment, TE suspected severe fibrosis, while ARFI showed only mild fibrosis. In one patient, global hepatic assessment and ARFI were concordant in the diagnoses liver cirrhosis, while TE detected no pathologic finding (Table 3).
Global hepatological assessment
For a global hepatic classification experienced hepatologists assessed all examinations, including liver stiffness measurements, serologic parameters and fibrotic markers in all patients. Globally, hepatic pathology was suspected in 17/24 patients (71%). Liver fibrosis was suspected in 11 patients (46%), and liver cirrhosis in six patients (25%) (Table 4).

Full table
Discussion
Over the last years it became important for clinicians who care for ACHD not only to understand the typical sequel of specific treated CHD, but also to develop a better understanding of non-cardiac comorbidities which may develop over the years in adulthood. Up to know the main research focused on Fontan-associated liver disease, showing consistently near-universal fibrosis both early and late after Fontan circulation.
So far, the prevalence and the extent of liver disease in Senning and Mustard patients remains unknown, and no data exist about the significance of a systematic screening. So much surprising were our results, demonstrating a liver involvement in more than two-thirds of our study cohort. Especially against the background that 92% of our patients were in NYHA class I/II, which represents a patient cohort in an extraordinary good condition compared to studies reporting about significant morbidity in the long-term follow up of atrial switch patients (3,5) with a depressed RV function and worse NYHA class (4).
Chronic liver impairment in TGA-ASO patients can be caused by an elevated systemic venous pressure due to stenosis in the systemic venous baffle. None of our patients, however, showed clinical or echocardiographic signs of baffle obstruction.
Another reason for liver impairment can be a reduced cardiac output, predominantly caused by failure of the morphologic RV in systemic position. In the present study, qualitative echocardiographic assessment revealed normal to mildly depressed systemic ventricle function. No patient had secondary pulmonary hypertension due to tricuspid valve insufficiency or congestive heart failure. It should be noted that only few patients with TGA-ASO will develop failure of the morphologic left ventricle in subpulmonary position. There was no significant mitral valve insufficiency correlating to the left ventricular dysfunction in subpulmonary position. No patient suffered from hemodynamically relevant rhythm disorders.
Hepatotoxic drugs, acute or chronic hepatitis and chronic alcohol abuse could be ruled out and were not responsible for liver alterations in our study population.
Liver stiffness is directly influenced by central venous pressure (3,29). Eicken and co-workers (3,30) investigated Senning and Mustard patients invasively in settings with volume load, atrial pacing and dobutamine stress. In summary, TGA-ASO patients were not able to increase cardiac output and their stroke volume adequately compared to normal controls. It was reasoned that non-compliant and stiff baffles lead to an absent buffering chamber function, limiting the biventricular preload and being causative for diastolic inflow impairment (3,31,32).
We postulate that in long-term course—as a consequence of the Senning and Mustard procedure—the stiffness of the baffles and the severely reduced atrial functions as contracting and relaxing compartments may lead to chronic congestion of the liver parenchyma, which we can detect as a fibrotic or even cirrhotic alteration.
We found no relevant differences in Senning and Mustard patients (with their baffles created of Goretex or pericardium, respectively) except in NTproBNP levels, which have to be interpreted carefully due to only few valid values.
Screening for liver disease in asymptomatic patients after atrial switch operations seems to be meaningful. Although specific serum markers of liver fibrosis were reasonable in some of our ACHD patients, we could not rely on these findings. Most of the biochemical markers have been validated to predict cirrhosis in patients with hepatitis and may not be useful for hepatic screening in patients with ACHD (6). Therefore, baseline laboratory tests should provide an indication of hepatic disease, but are unreliable in our ACHD population as single screening tool for hepatic involvement in patients after atrial switch operations (33-35).
Most of the Senning and Mustard patients with liver cirrhosis presented with increased GGT levels, but only 25% had elevated total bilirubin and two patients (8%) presented with slightly elevated transaminases. These results were not surprising while correlations of serologic liver parameters and histologic findings in the literature are inconsistent: older studies showed no coherence (3,17,28), while others revealed a correlation between serum transaminases, bilirubin, reduced cardiac output and elevation of venous pressures (36).
Although some specific serum markers are known to have the power to predict the severity of fibrosis, none of our patients had increased levels. Their specificity, however, may be reduced by other organ conditions (3,37).
Our 2D abdominal ultrasound results showed a poor sensitivity of 50%, but a specificity of 100% when compared to the results of ARFI and the overall clinical judgment of the hepatologist. These findings are not unusual and in concordance to the literature of hepatitis studies (3,38).
Specific ultrasound based procedures (TE and ARFI) are well established (3,39) in special clinical pictures of adults with CHD to detect liver pathologies, especially in Eisenmenger patients (3,18), and much more frequently in Fontan patients (3,24,28). Pfeiffer and co-workers compared ARFI and B-mode ultrasound with mini-laparoscopic liver biopsy. ARFI was found to have a good accuracy for the detection of significant fibrosis and cirrhosis compared to biopsy (3,38).
In the present study, we relied on TE and ARFI, which were each applicable in 83% of the subjects, while both measurements in combination offered pathological results in 16/24 patients (67%). Thereof, in 81% TE and ARFI provided identical or similar results (3,38).
MRI and CT imaging of the liver would have been excellent diagnostic tools (40), but our study concept was to use primary inexpensive and widely available diagnostic tools like baseline laboratory tests, abdominal ultrasound and easy performable liver stiffness measurements. Beside this, Mustard/ Senning patients often have a pacemaker due to sick-sinus syndrome and therefore no MRI examination is feasible.
For a liver screening, the combination of several methods seems to be crucial in order to identify liver disease early. Although we focused on cardiac abnormalities affecting the liver, and patients with primary liver diseases potentially affecting the heart were excluded, it should not be forgotten that liver cirrhosis can also exert a negative influence on the pulmonary and myocardial function. In our study population, however we had no signs for hepatopulmonary syndrome, portopulmonary hypertension or cirrhotic cardiomyopathy, respectively.
Since both, the cardiological and the hepatological aspects, are very specific, it is crucial to support an interdisciplinary care in experienced centers, where a multidisciplinary approach with a closed collaboration is assured to optimize the management of the ACHD population.
Study limitations
One drawback is the small number of Senning and Mustard patients in this study. Enrollment of the patients in only two tertiary centres for ACHD disease as well as the lack of a control group is further study limitations. Beside this, liver biopsy was omitted due to the unacceptably high risk in the study population.
Conclusions
The present study has shown that adults after Senning or Mustard operations are at risk of developing liver impairment. Therefore, we recommend to check the “liver status” on a regular basis while the time intervals depends on the level of impairment.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by regional ethics committees of the Technical University of Munich (No. 2503/09) and Charité Berlin and informed consent was taken from all the patients. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Senning A. Surgical correction of transposition of the great vessels. Surgery 1959;45:966-80. [PubMed]
- Mustard WT, Keith JD, Trusler GA, et al. The Surgical Management of Transposition of the Great Vessels. J Thorac Cardiovasc Surg 1964;48:953-8. [PubMed]
- Cuypers JA, Eindhoven JA, Slager MA, et al. The natural and unnatural history of the Mustard procedure: long-term outcome up to 40 years. Eur Heart J 2014;35:1666-74. [Crossref] [PubMed]
- Couperus LE, Vliegen HW, Zandstra TE, et al. Long-term outcome after atrial correction for transposition of the great arteries. Heart 2019;105:790-6. [Crossref] [PubMed]
- Dennis M, Kotchetkova I, Cordina R, et al. Long-Term Follow-up of Adults Following the Atrial Switch Operation for Transposition of the Great Arteries - A Contemporary Cohort. Heart Lung Circ 2018;27:1011-7. [Crossref] [PubMed]
- Lui GK, Saidi A, Bhatt AB, et al. Diagnosis and Management of Noncardiac Complications in Adults With Congenital Heart Disease: A Scientific Statement From the American Heart Association. Circulation 2017;136:e348-92. [Crossref] [PubMed]
- Correale M, Tarantino N, Petrucci R, et al. Liver disease and heart failure: Back and forth. Eur J Intern Med 2018;48:25-34. [Crossref] [PubMed]
- Alonso-Gonzalez R. Liver dysfunction and congenital heart disease: Are we ready for the epidemic? Int J Cardiol 2017;249:169-70. [Crossref] [PubMed]
- Johnson JA, Cetta F, Graham RP, et al. Identifying predictors of hepatic disease in patients after the Fontan operation: a postmortem analysis. J Thorac Cardiovasc Surg 2013;146:140-5. [Crossref] [PubMed]
- Schwartz MC, Sullivan LM, Glatz AC, et al. Portal and sinusoidal fibrosis are common on liver biopsy after Fontan surgery. Pediatr Cardiol 2013;34:135-42. [Crossref] [PubMed]
- Surrey LF, Russo P, Rychik J, et al. Prevalence and characterization of fibrosis in surveillance liver biopsies of patients with Fontan circulation. Hum Pathol 2016;57:106-15. [Crossref] [PubMed]
- Wu FM, Jonas MM, Opotowsky AR, et al. Portal and centrilobular hepatic fibrosis in Fontan circulation and clinical outcomes. J Heart Lung Transplant 2015;34:883-91. [Crossref] [PubMed]
- Bae JM, Jeon TY, Kim JS, et al. Fontan-associated liver disease: Spectrum of US findings. Eur J Radiol 2016;85:850-6. [Crossref] [PubMed]
- Pundi K, Pundi KN, Kamath PS, et al. Liver Disease in Patients After the Fontan Operation. Am J Cardiol 2016;117:456-60. [Crossref] [PubMed]
- Lindsay I, Johnson J, Everitt MD, et al. Impact of liver disease after the fontan operation. Am J Cardiol 2015;115:249-52. [Crossref] [PubMed]
- Wu FM, Kogon B, Earing MG, et al. Liver health in adults with Fontan circulation: A multicenter cross-sectional study. J Thorac Cardiovasc Surg 2017;153:656-64. [Crossref] [PubMed]
- Asrani SK, Asrani NS, Freese DK, et al. Congenital heart disease and the liver. Hepatology 2012;56:1160-9. [Crossref] [PubMed]
- Mebus S, Nagdyman N, Kugel J, et al. Non-invasive assessment of liver changes in Eisenmenger patients. Int J Cardiol 2017;249:140-4. [Crossref] [PubMed]
- Sun W, Cui H, Li N, et al. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: A meta-analysis study. Hepatol Res 2016;46:862-70. [Crossref] [PubMed]
- Kim BK. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010;30:546-53. [Crossref] [PubMed]
- Veillon P, Gallois Y, Moal V, et al. Assessment of new hyaluronic acid assays and their impact on FibroMeter scores. Clin Chim Acta 2011;412:347-52. [Crossref] [PubMed]
- DRG Instruments GmbH. In: EIA-5108 UsMCE, editor. Germany.
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846-54. [Crossref] [PubMed]
- Friedrich-Rust M, Koch C, Rentzsch A, et al. Noninvasive assessment of liver fibrosis in patients with Fontan circulation using transient elastography and biochemical fibrosis markers. J Thorac Cardiovasc Surg 2008;135:560-7. [Crossref] [PubMed]
- Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006;55:403-8. [Crossref] [PubMed]
- Lupsor M, Badea R, Stefanescu H, et al. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis 2009;18:303-10. [PubMed]
- Fierbinteanu-Braticevici C, Andronescu D, Usvat R, et al. Acoustic radiation force imaging sonoelastography for noninvasive staging of liver fibrosis. World J Gastroenterol 2009;15:5525-32. [Crossref] [PubMed]
- Melero-Ferrer JL, Osa-Saez A, Buendia-Fuentes F, et al. Fontan Circulation in Adult Patients: Acoustic Radiation Force Impulse Elastography as a Useful Tool for Liver Assessment. World J Pediatr Congenit Heart Surg 2014;5:365-71. [Crossref] [PubMed]
- Millonig G, Friedrich S, Adolf S, et al. Liver stiffness is directly influenced by central venous pressure. J Hepatol 2010;52:206-10. [Crossref] [PubMed]
- Eicken A, Michel J, Hager A, et al. Limited Ventricular Preload is the Main Reason for Reduced Stress Reserve After Atrial Baffle Repair. Pediatr Cardiol 2017;38:353-61. [Crossref] [PubMed]
- Derrick GP, Narang I, White PA, et al. Failure of stroke volume augmentation during exercise and dobutamine stress is unrelated to load-independent indexes of right ventricular performance after the Mustard operation. Circulation 2000;102:III154-9. [Crossref] [PubMed]
- Reich O, Voriskova M, Ruth C, et al. Long-term ventricular performance after intra-atrial correction of transposition: left ventricular filling is the major limitation. Heart 1997;78:376-81. [Crossref] [PubMed]
- Ofei SY, Gariepy C, Hanje J, et al. Liver fibrosis in adults with Fontan palliation: Do common screening studies predict disease severity? Int J Cardiol 2015;181:174-5. [Crossref] [PubMed]
- Baek JS, Bae EJ, Ko JS, et al. Late hepatic complications after Fontan operation; non-invasive markers of hepatic fibrosis and risk factors. Heart 2010;96:1750-5. [Crossref] [PubMed]
- Wu FM, Earing MG, Aboulhosn JA, et al. Predictive value of biomarkers of hepatic fibrosis in adult Fontan patients. J Heart Lung Transplant 2017;36:211-9. [Crossref] [PubMed]
- Naschitz JE, Slobodin G, Lewis RJ, et al. Heart diseases affecting the liver and liver diseases affecting the heart. Am Heart J 2000;140:111-20. [Crossref] [PubMed]
- Fallatah HI. Noninvasive Biomarkers of Liver Fibrosis: An Overview. Advances in Hepatology 2014;2014:15. [Crossref]
- Pfeifer L, Zopf S, Siebler J, et al. Prospective Evaluation of Acoustic Radiation Force Impulse (ARFI) Elastography and High-Frequency B-Mode Ultrasound in Compensated Patients for the Diagnosis of Liver Fibrosis/Cirrhosis in Comparison to Mini-Laparoscopic Biopsy. Ultraschall Med 2015;36:581-9. [Crossref] [PubMed]
- Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013;33:1138-47. [Crossref] [PubMed]
- Horvat N, Rocha MS, Chagas AL, et al. Multimodality Screening of Hepatic Nodules in Patients With Congenital Heart Disease After Fontan Procedure: Role of Ultrasound, ARFI Elastography, CT, and MRI. AJR Am J Roentgenol 2018;211:1212-20. [Crossref] [PubMed]


