Exercise stress echocardiography in patients with aortic stenosis: impact of baseline diastolic dysfunction and functional capacity on mortality and aortic valve replacement
Introduction
The prevalence of aortic stenosis (AS) due to degenerative valvular disease has been steadily increasing, and is the leading cause for valve replacement (1-3). Current guidelines recommend yearly resting transthoracic echocardiograms to assess for disease progression in asymptomatic patients with AS (Class 1), and exercise stress testing for the presence of exercise-induced symptoms and/or abnormal blood pressure responses (Class 2b) (4). Exercise stress echocardiography is also performed on patients with mild or moderate AS when symptoms are out of proportion to the disease, or to rule out coronary artery disease, which is prevalent in this cohort.
In significant AS, the chronic systolic pressure overload leads to adaptive remodeling of the heart including left ventricular hypertrophy, decreased compliance and diastolic dysfunction (DD). We hypothesized that worsening DD could impact functional capacity (FC), which is a known predictor of mortality and the need for aortic valve replacement (AVR) (5). There are limited data however on the interaction of DD with AS in relation to long-term outcomes. Therefore, the focus of this study is to assess the impact of DD on FC as well as the independent role of DD and FC in predicting long-term outcomes (all-cause mortality or the need for AVR) in patients with any degree of AS undergoing exercise stress echocardiogram.
Methods
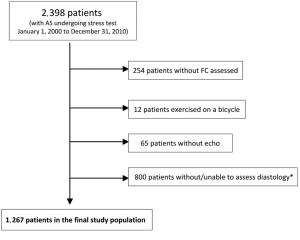
This is a retrospective analysis of prospectively collected data in a registry. The cohort consisted of consecutive patients with any degree of AS who underwent an outpatient stress echocardiogram for various reasons at the Cleveland Clinic between January 1, 2000 and December 31, 2010. Patients were identified using the stress database for all patients with any degree of AS [mild (peak velocity 2.6-2.9 m/s), moderate (3-4 m/s), and severe (>4 m/s)]. For patients with multiple studies, we included the first study performed and excluded subsequent studies. Patients were excluded if they underwent pharmacologic stress, did not exercise as part of the test, exercised on a bicycle, underwent nuclear perfusion images, did not undergo echocardiography, or had no assessment of FC or diastolic function. Additionally, patients with severe mitral stenosis (mean gradient ≥10 mmHg, valve area <1 cm2, N=8), severe mitral regurgitation (N=6), and severe aortic insufficiency (N=22) were excluded (Figure 1).
Diastolic function was assessed in a standardized method and in accordance with published guidelines using a combination of echocardiographic variables including transmitral inflow pattern, pulmonary venous flow pattern, and left atrial size (6). Diastolic function was labeled as normal, mild (grade I, impaired relaxation), moderate (grade II, pseudo-normal), or severe (grade III, restrictive) (7). Systolic function was assessed by quantitative and/or visual evaluation of the LVEF in accordance with published guidelines (8). LV mass and LV mass index were calculated (9,10). AS was assessed using peak transaortic jet velocity, mean transaortic gradient, and aortic valve area assessed by continuity equation. All echocardiograms were interpreted by experienced and board certified readers. The interobserver agreement of reproducibility and DD classification extrapolated from our ongoing quality assurance effort in our imaging core lab was on average 83%, and intraobserver agreement was 94%.
All exercise stress tests at our institution were performed under the supervision of a licensed exercise physiologist and physician. Exercise protocols utilized included the Cornell, Bruce, Naughton protocols as well as modifications of these protocols. Serial blood pressure and heart rate (HR) measurements were recorded during stress testing in addition to measurements of ischemia, development of arrhythmias, maximal metabolic equivalents (METs) achieved, and heart rate recovery (HRR) defined as the difference in HR between peak exercise and 1 minute into recovery.
The Cleveland Clinic stress database is an ongoing database that includes all stress tests performed at our institution. Patient variables (demographics, past medical history, medications, type of stress test, METs, and imaging data) were prospectively collected by trained personnel. Data were extracted for analysis and linked to the social security death index and the Cleveland Clinic Cardiovascular Information Registry (CVIR), a prospectively collected registry of all surgeries performed at our institution. The study was approved by the Cleveland Clinic institutional review board.
The primary endpoint was the combined endpoint of death or need for AVR. Secondary endpoints were the individual components of the primary endpoint. Death was defined as all-cause mortality (obtained using the Social Security Death Index with previously reported high specificity (11). Secondary endpoints were all-cause death and need for AVR.
Continuous data were expressed as a mean ± one standard deviation, and compared using the unpaired Student t-test or Wilcoxon rank test. Categorical data were displayed as frequencies and percentages, and comparisons were made using Chi-square tests or Fisher exact tests. All statistical tests were two sided. A P value <0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for Social Sciences, version 11.5, for Windows (SPSS, Chicago, Illinois). Linear regression was performed to identify factors associated with METs. Variables that were considered in this analysis were: age, gender, body mass index, diabetes, smoking, hypertension, prior myocardial infarction, prior coronary artery bypass surgery, prior AVR, mitral regurgitation, aortic regurgitation, mean aortic valve gradient, LVEF, left ventricular mass index, left atrial size, DD, HRR, and inducible ischemia. The strength of the model was expressed using Nagelkerke R-square. The analysis was repeated using METs as a dichotomous variable (≥7 or <7).
Kaplan-Meier curves were generated and compared using the log-rank test. Survival analysis treated the time of stress echocardiogram as ‘time 0’. The effect of METs and DD on outcomes was investigated using Cox regression analysis with proportional hazards model (CPH). The variables entered into the model for the primary outcome were: age, gender, systolic blood pressure, body mass index, left atrial size, left ventricular mass, LVEF, aorta size, aortic valve mean gradient, METs, HRR, type of stress test, diabetes, smoker, hyperlipidemia, prior myocardial infarction, prior CABG, prior AVR, diastolic function, mitral regurgitation, aortic regurgitation, and inducible ischemia. Interactions between DD and each of the following variables were individually assessed and entered into the final model: age, LVEF, diabetes, hypertension, and aortic valve mean gradient. Stepwise forward selection was used to create the final model. Models were carefully examined for the proportional hazards assumption, multicollinearity (such as peak and mean AV gradients; each was entered separately and the one yielding the strongest model was used), and the additive value of the terms. A ratio of 10 events per degree of freedom of the model was maintained for the primary outcome and whenever possible for the secondary outcomes. Subgroup analysis was performed for patients without history of coronary artery disease or prior AVR, and also for patients with moderate or severe AS.
In the death CPH model (secondary outcome), post-test AVR was entered as a time dependent covariate in the model. We also performed subgroup analysis by excluding patients with known CAD, prior CABG or prior AVR (N=408) and had 859 left for analysis.
Results
We identified 2,398 patients with various degrees of AS who underwent stress testing at our institution between January 1, 2000 and December 31, 2010. After all exclusion criteria were met, 1,267 patients were included in the analysis (Figure 1). The baseline characteristics are summarized in Table 1. Patients in our cohort were predominantly male (75%), had a mean age of 67±11 years, LVEF of (56±7)%, mean aortic valve gradient of 19±12 mmHg, and mean METs achieved of 8±2.6. The proportion of patients with normal, stage 1, and ≥ stage 2 DD was 195 (15%), 928 (73%), 144 (12%) respectively.

Full table
There were 475 (37.5%) patients who had the primary outcome (death or AVR) with 164 (12.9%) deaths at a mean follow up of 5.6±4.1 years (no deaths occurred within 30 days of stress testing) and 341 (27%) patients requiring AVR at a mean follow up of 2.0±2.6 years. There was no significant difference in the primary outcome among those included and excluded from the study (37.5% vs. 40.0%, P=0.17). The characteristics of patients who died and those had had AVR are summarized in Table 1.
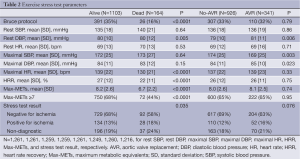
Table 2 shows stress test parameters stratified by outcome. Those who died were less likely to perform the Bruce protocol and achieved a lower HRR, maximum HR, METs, and percentage of those achieving acceptable FC as defined by a MET of at least 7. They were also more likely to have evidence of ischemia on stress test. Those who received AVR had a mildly higher, albeit statistically significant, diastolic blood pressure, and a lower systolic blood pressure.
Full table
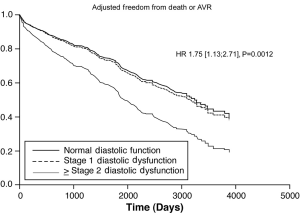
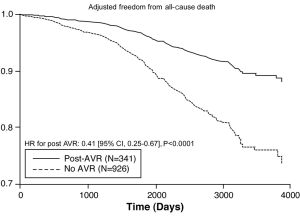
After adjusting for confounding effects, independent predictors of the composite end-point of death or AVR were AV mean gradient ascending aorta size, stage ≥ II DD, prior AVR, METS, and HRR (Table 3). Figure 2 demonstrates the relationship between severity of DD and the composite endpoint. The independent predictors for secondary outcomes are summarized in Tables 4,5. Finally, patients who had AVR had significantly lower mortality than those who did not undergo AVR (adjusted HR 0.41, P<0.0001) (Figure 3).
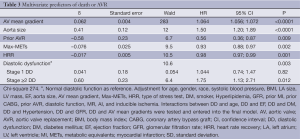
Full table
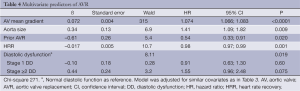
Full table

Full table
FC was measured by the METs achieved during exercise stress echocardiography. Independent negative predictors of FC were older age, female gender, higher body mass index, non-Bruce protocol, lower mean aortic valve gradient, and inducible ischemia. Independent positive predictors of FC were higher HRR and LVEF. Baseline DD was not a predictor of METs achieved on exercise stress echocardiography (Table 6).
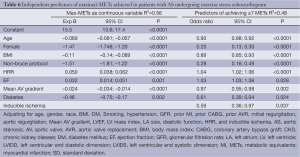
Full table
Subgroup analysis of patients without prior CAD or AVR
There were 859 patients with no prior CAD, prior CABG or AVR (156 with normal, 650 with stage 1, and 53 with stage ≥2 DD). DD was associated with poor FC [unadjusted OR 0.180 (0.11, 0.31) and 0.19 (0.09, 0.39) for predicting METS ≥7 with stage 1 and stage ≥2, respectively, P<0.0001], but was not an independent predictor after adjusting for other covariates. In addition, DD was associated with increased primary endpoint (unadjusted event rate: 36% vs. 41% vs. 51%, log-rank P=0.0012). After adjustment for demographics, comorbidities, echocardiographic parameters, stage 2 DD was still associated with increased outcomes [HR 1.90 (1.07, 3.37), P=0.028].
Subgroup analysis of patients with moderate or severe AS
After excluding patients with mild AS, there were 413 patients with moderate or severe AS. Advanced DD stage ≥2 was associated with lower odds of achieving METS ≥7 [unadjusted ratio 0.25 (0.15, 0.35)] but was not an independent predictor of FC after adjusting for confounders. Finally, advanced DD was associated with a trend for increased primary endpoint [HR 2.20 (0.98, 3.94)].
Discussion
In the current study, we demonstrated that in AS patients undergoing exercise echocardiography, baseline DD is not predictive of FC after multivariate adjustment. However, both baseline DD and FC are independent predictors of the combined endpoint of death or need for AVR and highlight additional parameters that might assist in identifying a high risk cohort of patients with all degrees of AS.
Despite current guidelines that recommend surgical intervention for patients with symptomatic severe AS meeting Class 1 indications (4,12), the management of asymptomatic severe AS is less clear. Given the low but not trivial morbidity and mortality associated with AVR (13,14), it is appropriate to risk stratify asymptomatic patients and identify those who would derive the most benefit from aggressive monitoring and treatment. Studies demonstrate that rapid progression, defined by an increase in aortic jet velocity >0.3 mg/second/year and/or a decrease in valve area >0.1 cm2/year may lead to adverse clinical outcomes (5,15,16). There is also evidence that asymptomatic patients with mild to severe AS and above age 50 with severe valve calcification and/or coronary artery disease have worse clinical outcomes (15,17). The safety and potential importance of exercise stress testing as a prognostic indicator in patients with asymptomatic severe AS has also been identified (18-20). A study of 125 patients with asymptomatic AS and found that those with an effective orifice area (EOA) of <1.2 cm2 with symptoms during exercise stress testing had a 79% chance of developing symptoms at 12 months, identifying a subset who would benefit from early intervention (18). Our study identifies DD as an additional parameter which can be used to identify those with all degrees of AS at higher risk of poor outcomes. Interestingly, only 13% of patients had advanced DD which is keep with a relatively asymptomatic cohort.
Given the reversibility of systolic dysfunction after surgical intervention (21), the importance of AVR for those with systolic dysfunction has been well established. However, the impact of DD on symptomatology and need for AVR has not been well defined. Several studies identified left ventricular hypertrophy as the most common cause of DD in AS patients, and might regresswith AVR (22-24). These findings are important given that DD is thought to be the major driverfor elevated left atrial and pulmonary artery systolic pressures in moderate to severe AS (25,26), and could therefore explain some of the experienced symptoms.
Contrary to our hypothesis, we did not find DD to be a statistically significant predictor of FC for patients with asymptomatic AS. This may indicate that those in whom DD contributes to a significant deterioration in FC are already symptomatic at baseline or that the symptoms resulting from DD were not limiting. This may also imply that DD causing significant elevation in filling pressures resulting in symptoms is only seen in those with higher degrees of AS. However, this study was not sufficiently powered to stratify FC by severity of AS given the smaller number of those with severe AS.
We did however identify DD as an independent predictor of the composite end-point of death and need for AVR. These findings are supported by a study of 36,261 patients where moderate or severe DD was associated with increased mortality (27). A relatively large study by Gjertsson et al. of 399 patients undergoing AVR found that moderate to severe DD was an independent predictor of mortality after AVR (28). A smaller study by Poh et al. of 53 asymptomatic AS patients found late diastolic filling to be a predictor of cardiac death and need for valve surgery (29). Our results suggest that while DD may not be an independent predictor of FC, it may be an early predictor of progression to symptomatic and/or hemodynamically significant AS that warrants intervention, particularly advanced stage ≥2. To our knowledge, this is the first large study in which DD has been implicated as an independent predictor of adverse outcomes in those with asymptomatic AS. The mechanisms by which this occurs is unclear but could be related to degree of left ventricular hypertrophy (30). Similarly, less desirable outcomes after AVR in patients with DD may be due to the association of DD with patient-prosthesis mismatch (31).
Previous studies identify FC as an important predictor of all-cause and cardiovascular mortality (32,33). Although the prognostic implications of FC have not been studied in asymptomatic AS patients, there is evidence that exercise time is a significant predictor of symptoms at 12 months (18). Our study shows that FC is an independent predictor of the composite end-point, and was predictive of mortality when multivariate analysis of AVR and mortality was done independently. These results strengthen the argument for exercise stress testing in patients with various degrees of AS.
This study highlights the importance of stress echocardiogram in risk stratifying patients with AS by providing both diastology and FC parameters. The change of diastolic grade and filling pressures at peak exercise may also be more predictive of outcomes and warrant further investigation. However, in asymptomatic patients with severe AS, the presence of advanced DD at rest echocardiogram might be an indication for early surgery and should prompt early intervention. This needs to be validated and tested prospectively in large randomized clinical trials.
Strengths and limitations
This is the largest study to our knowledge to assess the interaction between DD, various degrees of AS, FC and long-term outcomes. However, we acknowledge several limitations. This is a retrospective study from a single tertiary center, with likely referral and selection bias. The true indication for each stress test and whether patients were asymptomatic or not was not well defined or available in the database. The characteristics of AS patients who did not undergo stress testing are unknown. The majority of the patients in our cohort had mild and moderate AS. Therefore, our study sample was not powered to stratify outcomes for severe AS patients; still we did a subgroup analysis and showed that DD was still predictive of events. However, including patients with all degrees of AS may be a strength of the analysis as the full spectrum of the disease was covered. While aortic valve gradients and peak systolic velocities are used to classify the severity of AS, patients may have severe AS with low gradients (due to a small LV stroke volume or decreased LVEF) making dimensionless index (not available in the database) another helpful parameter. This may not have significantly affected the results given the majority of patients had preserved LVEF. Furthermore, our primary endpoint was a composite of AVR and mortality, implying that AS was the driving factor for mortality of these patients. While a limitation, this composite end-point has been used in the majority of other papers on the topic. We were also unable to identify patients who underwent AVR at another hospital. In addition, DD is not a static but rather a dynamic condition (34), and DD classifications and definitions have changed over time. Data on E/e’was not available in the database for a large proportion of patients and therefore not included in the analysis. DD at the time of the AVR or post-surgically was not known as it was only measured at baseline. We did not evaluate the effects of exercise on DD or peak right ventricular systolic pressure, two possible predictors of outcomes, as these values were not reported until recently. We also did not have data on maximal oxygen consumption, development of hypotension during stress, or degree of valve calcification by echo, which are known predictors of outcomes.
Conclusions
This study aimed to assess the relationship between FC, DD, and outcomes in patients with various degrees of AS undergoing exercise echocardiography. Although, DD was not predictive of FC as defined by METs achieved, we did demonstrate that FC and DD are independent predictors of the composite end-point of death or need for AVR. These findings highlight additional parameters that could assist in identifying and managing a high risk cohort of patients with all degrees of AS. The presence of advanced DD in otherwise asymptomatic patients with severe AS might be an additional parameter that triggers the decision for early intervention. Further investigation and prospective clinical trials are warranted to validate these findings as these could have implications on the decision to operate for those with severe AS and on intensity of screening for those with mild and moderate disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [PubMed]
- Ozkan A, Kapadia S, Tuzcu M, et al. Assessment of left ventricular function in aortic stenosis. Nat Rev Cardiol 2011;8:494-501. [PubMed]
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [PubMed]
- Association Task Force on Practice Guidelines, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006;114:e84-231. [PubMed]
- Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997;95:2262-70. [PubMed]
- Quiñones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 2002;15:167-84. [PubMed]
- Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta Stone. J Am Coll Cardiol 1997;30:8-18. [PubMed]
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440-63. [PubMed]
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450-8. [PubMed]
- de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 1992;20:1251-60. [PubMed]
- Lauer MS, Blackstone EH, Young JB, et al. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol 1999;34:618-20. [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [PubMed]
- Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol 2001;37:885-92. [PubMed]
- Banbury MK, Cosgrove DM 3rd, White JA, et al. Age and valve size effect on the long-term durability of the Carpentier-Edwards aortic pericardial bioprosthesis. Ann Thorac Surg 2001;72:753-7. [PubMed]
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611-7. [PubMed]
- Lancellotti P, Magne J, Donal E, et al. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol 2012;59:235-43. [PubMed]
- Rosenhek R, Klaar U, Schemper M, et al. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J 2004;25:199-205. [PubMed]
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005;26:1309-13. [PubMed]
- Clyne CA, Arrighi JA, Maron BJ, et al. Systemic and left ventricular responses to exercise stress in asymptomatic patients with valvular aortic stenosis. Am J Cardiol 1991;68:1469-76. [PubMed]
- Alborino D, Hoffmann JL, Fournet PC, et al. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis 2002;11:204-9. [PubMed]
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005;91:1324-9. [PubMed]
- Hess OM, Villari B, Krayenbuehl HP. Diastolic dysfunction in aortic stenosis. Circulation 1993;87:IV73-6. [PubMed]
- Guarracino F, Talini E, Landoni G, et al. Effect of aortic valve surgery on left ventricular diastole assessed by echocardiography and neuroendocrine response: percutaneous versus surgical approach. J Cardiothorac Vasc Anesth 2010;24:25-9. [PubMed]
- Murakami T, Hess OM, Gage JE, et al. Diastolic filling dynamics in patients with aortic stenosis. Circulation 1986;73:1162-74. [PubMed]
- Casaclang-Verzosa G, Nkomo VT, Sarano ME, et al. E/Ea is the major determinant of pulmonary artery pressure in moderate to severe aortic stenosis. J Am Soc Echocardiogr 2008;21:824-7. [PubMed]
- Yong G, Ali A, Feldman T. Diastolic transmitral valve pressure gradients in patients with severe calcific aortic stenosis. Catheter Cardiovasc Interv 2009;74:957-64. [PubMed]
- Halley CM, Houghtaling PL, Khalil MK, et al. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med 2011;171:1082-7. [PubMed]
- Gjertsson P, Caidahl K, Farasati M, et al. Preoperative moderate to severe diastolic dysfunction: a novel Doppler echocardiographic long-term prognostic factor in patients with severe aortic stenosis. J Thorac Cardiovasc Surg 2005;129:890-6. [PubMed]
- Poh KK, Chan MY, Yang H, et al. Prognostication of valvular aortic stenosis using tissue Doppler echocardiography: underappreciated importance of late diastolic mitral annular velocity. J Am Soc Echocardiogr 2008;21:475-81. [PubMed]
- Hanayama N, Christakis GT, Mallidi HR, et al. Determinants of incomplete left ventricular mass regression following aortic valve replacement for aortic stenosis. J Card Surg 2005;20:307-13. [PubMed]
- Brown J, Shah P, Stanton T, et al. Interaction and prognostic effects of left ventricular diastolic dysfunction and patient-prosthesis mismatch as determinants of outcome after isolated aortic valve replacement. Am J Cardiol 2009;104:707-12. [PubMed]
- Lauer MS, Pashkow FJ, Snader CE, et al. Gender and referral for coronary angiography after treadmill thallium testing. Am J Cardiol 1996;78:278-83. [PubMed]
- Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793-801. [PubMed]
- Aljaroudi W, Alraies MC, Halley C, et al. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation 2012;125:782-8. [PubMed]