
Case report: assessment and management of myocardial infarction and non-obstructive coronary arteries (MINOCA): the role of microvascular coronary vasospasm
Introduction
Cardiovascular disease is leading cause of death in women predominantly due to ischemic heart disease (1). Women presenting with signs and symptoms of myocardial ischemia are more likely than men to have non-obstructive coronary artery disease (CAD) (2). Myocardial infarction with non-obstructive CAD (MINOCA) is clinically defined as acute myocardial infarction in the absence of (≥50% stenosis) obstructive CAD in any artery (3). The Women’s Ischemic Syndrome Evaluation (WISE) study at 10-year follow-up demonstrated that women with no obstructive CAD were at increased risk of death or nonfatal myocardial infraction (MI) (2). MINOCA is a working diagnosis that has been increasingly described to have likely overlap with mechanisms contributing to ischemia with non-obstructive coronary artery disease (INOCA) such as Prinzmetal variant angina (coronary spasm), coronary microvascular disorders, coronary plaque rupture, Takotsubo cardiomyopathy, and or coronary emboli (4). Coronary artery spasm has been notably proposed to be one of the underlying causes of MINOCA (3). Since traditional coronary angiography is likely to miss identifying the etiology behind the myocardial injury in these patients, several diagnostic tests can be useful such as invasive coronary reactivity testing (CRT), non-invasive positron emission tomography with coronary flow reserve (CFR), and/or myocardial perfusion on a stress cardiac magnetic resonance imaging (CMRI). Intracoronary (IC) pharmacologic testing may provide to be a valuable tool for establishing the diagnosis of functional abnormality underlying the symptoms of INOCA patients and lead to understanding the importance of role of coronary microvascular spasm (5). We are reporting a case of MINOCA due to coronary microvascular spasm diagnosed by invasive CRT as part of Women’s Ischemia Syndrome Evaluation Coronary Vascular Dysfunction (WISE-CVD) Continuation study (NCT02582021).
Case presentation
Initial presentation
A 55-year-old female with a past medical history of hypertension and long-standing history of migraines and angina self-referred to a cardiologist in 2007 due to symptoms of persistent angina. She underwent an exercise treadmill stress test, which showed normal results. Eight years later, patient subsequently presented to the emergency room after experiencing 45 minutes of persistent angina (level 6–7/10) radiating to both shoulders and intermittent to the left arm, nausea and diaphoresis with a blood pressure of 141/79 mmHg and heart rate of 59 beats per minute. Electrocardiogram (ECG) demonstrated sinus bradycardia, poor R wave progression, with no ischemic ST/T abnormalities. Initial troponin I was elevated at 0.40 ng/mL (reference range ≤0.04 ng/mL). Patient was evaluated for non-ST elevation myocardial infarction, with a peak troponin I level of 1.81 ng/mL. She was taken for coronary angiography which was reported as normal, with only mild atherosclerotic disease of the left anterior descending artery (LAD) and no signs of obstructive coronary artery disease, coronary dissection, bridging or embolism. IC imaging was not performed. Patient also had an echocardiogram that showed normal left ventricular size, wall thickness, wall motion, function (ejection fraction of 60%) and trace aortic regurgitation. Hypercoagulable workup was negative. Patient was discharged home to follow up as an outpatient on atorvastatin 20 mg daily, valsartan 320 mg daily, metoprolol 25 mg twice a day, and sublingual nitroglycerin (SL-NTG) as needed.
Outpatient evaluation
Due to her persistent symptoms, she presented to our tertiary care center for further evaluation approximately 2 weeks after her MINOCA. She had nocturnal angina which was relieved by sublingual nitroglycerin, as well as some episodes of exertional angina and dyspnea while walking uphill. Her Seattle Angina Questionnaire (SAQ) score was 97.2 for physical limitation, 50 for angina stability, 60 for angina frequency, 58.3 for quality of life, and 93.75 for treatment satisfaction (6). During her initial consult, her blood pressure was 135/90 mmHg, pulse was 61 beats per minute, and body mass index (BMI) was 22 kg/m2 with an otherwise unremarkable physical exam. ECG demonstrated normal sinus rhythm with no Q-waves but nonspecific T wave abnormality. Patient was discontinued from metoprolol and prescribed carvedilol, given concern for exacerbating suspected coronary spasm. She was also started on aspirin given her history of MINOCA. Other recommendations included the tapering of hormone replacement therapy and referral to cardiac rehabilitation. Due to the persistent symptoms in the setting of non-obstructive CAD, she underwent invasive CRT for diagnosis and identification of treatment targets.
CRT
The patient underwent invasive CRT, and all vasoactive medications and caffeine were withheld for 24–48 hours prior to the test per protocol as previously published (7). Baseline coronary angiography demonstrated mild atherosclerotic disease of the LAD, with otherwise normal epicardial coronary arteries and no evidence of obstructive coronary artery disease, coronary dissection or myocardial bridging. Transient ECG changes occurred during Doppler wire placement accompanied by angina and no significant epicardial vasospasm on coronary angiography, consistent with microvascular spasm (Figure 1). Her CFR in response to 18 mcg IC bolus of adenosine was 5.8 (abnormal <2.5), indicating supra-normal endothelium-independent coronary microvascular function (Table 1). The endothelium-dependent coronary epicardial function was borderline normal with 2.2% (abnormal ≤0%) increase in diameter response to 36.4 mcg of IC-acetylcholine (ACh). Coronary blood flow in response to 36.4 mcg of IC ACh increased by 43% (abnormal <50%), indicating abnormal endothelial-dependent coronary microvascular dysfunction (CMD). Testing for epicardial coronary vasospasm with 108 mcg of IC ACh revealed mild diffuse vasoconstriction of the LAD with a quantitative 9% reduction in diameter, along with angina and ST depression, indicating the presence of coronary microvascular vasospasm but no macrovascular vasospasm. There was a normal dilation smooth muscle response to 200 mcg of IC nitroglycerin, with resolution of angina and ST abnormalities. Based on the findings above, patient was diagnosed with coronary endothelium-dependent microvascular dysfunction and coronary microvascular vasospasm.
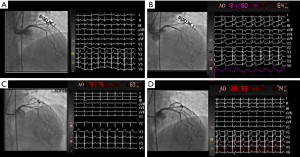
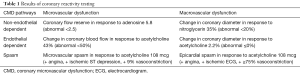
Full table
CMRI
The patient also underwent CMRI with perfusion imaging at rest and with adenosine stress (140 µg/kg per minute) nine months after her MINOCA. The results of her CMRI conducted for the registry research showed normal biventricular function with left ventricular ejection fraction 62%, no evidence of left ventricular remodeling with left ventricular mass/volume ratio 0.7 g/mL, normal regional wall motion, no visual late gadolinium enhancement or first-pass perfusion defects on qualitative analysis. Semi-quantitative analysis of microvascular perfusion revealed a low mean global transmural myocardial perfusion reserve index (MPRI) of 1.76, in which MPRI 1.84 predicts CMD in women with INOCA (8).
Treatment
Following these findings, medical therapy was adjusted. Carvedilol was gradually tapered off and long-acting diltiazem 120 mg daily was initiated for the treatment of coronary microvascular vasospasm prior to discharge. She continued to have symptoms with diltiazem and was started on amlodipine 5 mg daily with significant improvement. SL-NTG was continued as needed for episodes of angina. Valsartan, aspirin, and atorvastatin were continued for the treatment of hypertension and endothelial dysfunction. Estrogen-based hormonal therapy was tapered, and patient was referred to cardiac rehabilitation. At 18-month follow-up, the subject reported responding well to this treatment, with reduced frequency of angina occurring every few weeks.
Discussion
This case of MINOCA and persistent angina in a patient found to have coronary microvascular spasm highlights the importance of definitive diagnostic testing for coronary vasomotor dysfunction. Identification of the underlying causes of MINOCA represent a diagnostic and therapeutic challenge to clinicians as there are no current appropriate management guidelines established for these patients. Prior studies have demonstrated that MINOCA represents 6–14% of all acute MI cases and MINOCA is also associated with an all-cause mortality rate of 4.7% at 12-month follow up (9,10). Causes of MINOCA include myocarditis, occlusive or cocaine induced coronary spasm or thrombosis, plaque erosion, Takotsubo cardiomyopathy, spontaneous coronary artery dissection, and genetic hypercoagulable disorders (3).
MINOCA continues to be a therapeutic challenge as patients may exhibit a different cardiovascular risk profile than obstructive CAD patients. Current methods of treatment include targeting known cardiovascular risk factors and prescribing angiotensin-converting enzyme inhibitors and beta-blockers to alleviate symptoms associated with myocardial ischemia (9). MINOCA has been found to be more prevalent in women and younger adults and affect patients who report a higher frequency of hypertension than a diagnosis of hyperlipidemia or diabetes (10). Further evaluating the mechanisms of potential underlying causes, such as coronary spasm, should be regularly considered as a differential diagnosis as it may provide important clinical implications for future treatment.
Coronary spasm, particularly microvascular spasm, is often an important etiology of angina that is often overlooked and undiagnosed. Coronary microvascular spasm is defined as ischemic ECG changes (i.e., ST-segment depression or ST-segment elevation of 0.1 mV or T-wave peaking in at least 2 contiguous leads) in the absence of epicardial coronary spasm (75% diameter reduction with reproducible symptoms of the patient) (11). Prior reports have shown that severe attacks of coronary spasm may progress to myocardial infarction or sudden death (12). Coronary spasm is not only associated with a fixed atherosclerotic lesion but may also present in patient with normal arteries or minimal atherosclerosis (11). Due to the transient nature of coronary vasospasm and its response to nitroglycerin, ECGs and coronary angiography may often appear normal at the time of evaluation. In addition, noninvasive testing may also appear normal in patients with symptoms of ischemia but no obstructive coronary arteries (13).
Invasive CRT using vasoactive agents to evaluate macro and microvascular responses is considered the gold standard for definitive diagnosis of CMD and has been shown by the CASPAR (Coronary Artery Spasm in Patients with ACS) study to have no clinical adverse events due to injecting incremental doses of ACh (14). ACh provocation testing has proven to be useful to diagnose both coronary spasm and epicardial vasospasm in patients with exertional anginal symptoms in the ACOVA (Abnormal Coronary Vasomotion in patients with stable angina and unobstructed coronary arteries) study, with coronary microvascular spasm found in 55% of the patients studied (5). Although provocative testing may be effective, it may not as frequently be performed as it requires properly equipped facilities, experienced physicians, and the appropriate patient population presenting with suspected symptoms of coronary spasm. Identification of underlying causes of MINOCA using a complete diagnostic work-up is important not only for knowing its prognostic implications but also for determining specific targeted therapies.
Conclusions
This case presentation evaluates a case of MINOCA secondary to coronary microvascular vasospasm in a woman diagnosed by using invasive CRT. Diagnostic strategies like CRT used for the detection of vasospasm in coronary microvascular disease can be incorporated for routine assessment of MINOCA, which can subsequently influence clinical management as was shown in this case presentation. Prospective clinical trials are needed to ascertain whether effective treatment of spasm improves symptoms and reduces adverse outcomes.
Acknowledgments
Funding: Research reported in this publication was supported by the National Heart, Lung and Blood Institute (NHLBI) under grant numbers N01HV68161, N01HV68162, N01HV68163, N01HV68164, U01HL64829, U01HL64914, U01HL64924, K23HL105787, T32HL69751, R01HL090957, R01HL33610, R01HL56921, and UM1HL087366; the National Institute on Aging (NIA) under grant number R03AG032631; the National Center for Research Resources (NCRR) under grant number M01RR000425; the National Center for Advancing Translational Sciences (NCATS) under grant numbers UL1TR000124, UL1TR000064, UL1TR001427. This work was also supported by grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ; The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA; The Society for Women’s Health Research (SWHR), Washington, D.C.; QMED, Inc., Laurence Harbor, NJ; The Women’s Guild of Cedars-Sinai, the Edythe L. Broad, the Constance Austin Women’s Heart Research Fellowships, the Barbra Streisand Women's Cardiovascular Research and Education Program, the Linda Joy Pollin Women’s Heart Health Program, the Erika J. Glazer Women’s Heart Research Initiative, and The Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, CA; the Gatorade Trust and the PCORnet-One Florida Clinical Research Consortium CDRN-1501-26692, University of Florida, Gainesville, FL.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for use data for academic publication purposes.
References
- Garcia M, Mulvagh SL, Merz CN, et al. Cardiovascular Disease in Women: Clinical Perspectives. Circ Res 2016;118:1273-93. [Crossref] [PubMed]
- Bairey Merz CN, Shaw LJ, Reis SE, et al. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 2006;47:S21-9. [Crossref] [PubMed]
- Agewall S, Beltrame JF, Reynolds HR, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143-53. [PubMed]
- Sharaf B, Wood T, Shaw L, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J 2013;166:134-41. [Crossref] [PubMed]
- Ong P, Athanasiadis A, Borgulya G, et al. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol 2012;59:655-62. [Crossref] [PubMed]
- Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333-41. [Crossref] [PubMed]
- Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv 2012;5:646-53. [Crossref] [PubMed]
- Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging 2015. [Crossref] [PubMed]
- Mehta PK, Bairey Merz CN. Angina in Subjects with Evidence of Myocardial Ischemia and No Obstructive Coronary Artery Disease. In: de Lomos J, Omland T. editors. Chronic Coronary Artery Disease: A Companion to Braunwald's Heart Disease. 1st ed. Philadelphia, PA: Elsevier; 2017:374-90.
- Pacheco Claudio C, Quesada O, Pepine CJ, et al. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease). Clin Cardiol 2018;41:185-93. [Crossref] [PubMed]
- Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J 2017;38:2565-8. [PubMed]
- Slavich M, Patel RS. Coronary artery spasm: Current knowledge and residual uncertainties. Int J Cardiol Heart Vasc 2016;10:47-53. [Crossref] [PubMed]
- Cassar A, Chareonthaitawee P, Rihal CS, et al. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv 2009;2:237-44. [Crossref] [PubMed]
- Ong P, Athanasiadis A, Hill S, et al. Coronary artery spasm as a frequent cause of acute coronary syndrome: The CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) Study. J Am Coll Cardiol 2008;52:523-7. [Crossref] [PubMed]


