
The Sapien valve provides enough grip to be implanted in pulmonary position without a pre-stent
Introduction
Transcatheter pulmonary valve implantation (TPVI) proved to be a safe and less invasive treatment option for dysfunctional right ventricle to pulmonary artery (RV-PA) conduits and for dysfunctional “native” right ventricular outflow tracts (RVOTs) (1-3). Since the first report in 2000 (4) technical improvement has facilitated valve implantation. Pre-stenting reduced the incidence of Melody valve fractures and is a widely used technique to create a reliable and solid landing zone for a percutaneous valve (5). In contrast to the Medtronic Melody valve, which showed a relatively high incidence of stent fractures if implanted without pre-stenting (6) the Edwards Sapien valve consists of a strong balloon expandable stent, designed for direct implantation into aortic position. This concept proved very successful and stent fractures are not frequently reported although thousands of patients have been treated with this valve worldwide in aortic position without pre-stenting (7,8). Especially modifications of the newer generation Sapien S3 (S3; Edwards Lifesciences, Inc., Irvine, CA, USA) with larger cells and wide strut angles, improved cuff system to for an enhanced sealing of the valve and the lower profile of the delivery system allow the treatment of more challenging anatomies. Although initially designed for the aortic position, we report on a TPVI case series with successful implantation of the Sapien XT/S3 valve without using a pre-stent, thereby shortening and facilitating the implantation procedure.
Methods
Study design and patient selection
This retrospective, single centre study reviewed the procedural outcomes of patients receiving the Sapien XT/S3 transcatheter valve in pulmonic position without pre-stenting. Implantation of a Sapien XT/S3 valve without a pre-stenting was considered feasible if a balloon-test revealed a waist, enough to firmly anchor the valve. All patients provided written informed consent for the procedure and data acquisition was approved by the local ethics committee.
Endpoints
Successful implantation of the Sapien XT or Sapien 3 was defined as appropriate positioning of the transcatheter valve into the proper anatomical location, intended performance of the valve with a peak to peak gradient between RV and PA of less than 20 mmHg and absence of valve regurgitation or paravalvular leakage documented by angiography.
Procedural steps
After hemodynamic measurement and angiography of the RV and pulmonary arteries a balloon test was performed with a non-compliant balloon. The balloon diameter was selected larger than the systolic diameter of the RVOT/central PA in order to unmask a narrowing/balloon waist. The presence of a waist with a 1–2-mm smaller diameter than the diameter of the fully inflated balloon was considered enough to serve as an anchor for the newly implanted valve. Contrast agent was applied simultaneously into the aortic root for coronary testing and through the long-sheath placed in the RVOT. If contrast passed aside from the inflated balloon, the landing zone was considered not stable enough to anchor the valve. The presence of a waist in combination with a complete sealed RVOT by the inflated sizing balloon were the major criteria for implantation of a Sapien valve without a pre-stent. The choice of the Sapien valve depended on the measured waist diameter. In general, the next larger available Sapien valve as the measured waist diameter has been selected. Rapid pacing was performed in order to minimize valve movement during deployment to prevent dislocation during balloon inflation. Pacing was achieved with a 3-Fr bi-pacing ball (VYGON GmbH & Co. KG, Germany) introduced into the left ventricle through a 5-Fr guiding catheter JR (CORDIS®, Milpitas, US). All valves have been implanted in combination with the corresponding delivery systems (Novaflex or Commander).
Results
Overall, 6 patients were successfully treated with the Sapien XT/S3 valve without pre-stenting. Indication for TPVI was in 2/6 (33.3%) of the patients a combined valve pathology with stenosis and regurgitation of the pulmonary valve. The remaining patients had predominant pulmonary regurgitation (PR). The demographic parameters are summarized in Table 1.
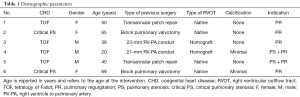
Full table
Procedural time from placement to removal of the sheaths comprised at median 2:32 hours. The median fluoroscopy time was 20:04 minutes. Representative results are presented in Figure 1. One of the patients (patient no. 6) developed ventricular tachycardia (VT) during the advancement of the valve into the final RVOT position due to mechanical tension caused by the stiff delivery system. The patient had a history of VT and an implantable cardioverter-defibrillator had been implanted which was inactivated temporarily to prevent shocks during rapid pacing. External defibrillation terminated the VT and the patient stabilized. Overall, no device malfunction, and no valve dislocation occurred during valve deployment.
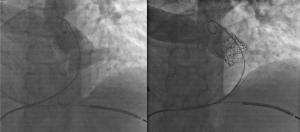
One patient showed minimal regurgitation of the Sapien valve on angiography after implantation. There were no signs of valve dysfunction or paravalvular leakage immediately after the implantation. Procedural data are summarized in Table 2. All patients received transthoracic echocardiography prior to hospital discharge showing a normal Doppler velocity flow signal across the implanted Sapien valve in the RVOT. Absence of a paravalvular leakage was documented in all patients. The median follow-up time was 6 months. Most of the patients received a cardiac magnetic resonance imaging (cMRI) at follow-up. In two of the patients (no. 5 and 6) information about late structural valve dysfunctions or paravalvular leakages was obtained by echocardiography.

Full table
Discussion
In the present case series implantation of the Sapien XT/S3 valve in pulmonary position was successful even the procedure was done without a pre-stent. The outer surface of the Sapien valve provides enough grip to secure the valve position in pulmonary position and to prevent a paravalvular leakage.
Currently, there are two different types of balloon expandable valves available for the pulmonic position: the Melody® (Medtronic Minneapolis, MN, USA) and the Sapien® XT (Edwards Lifesciences, Irvine, CA). Alternatively, the Sapien S3, a further development of the XT, is sometimes used in an off-label setting. For the Melody® valve a bovine jugular valve is sutured into the frame of a CP8Z34 Stent (NuMED, Inc., Hopkinton, NY, USA). The leaflets of the Sapien XT are of bovine pericardial tissue, sutured on a cobalt chromium frame and protected by a polyethylene terephthalate (PET) fabric skirt.
As the Melody valve received CE mark in 2006, stent fractures represented the most common early complication after the implantation of a Melody valve (6). Implantation of a pre-stent was therefore introduced and proved beneficial for the incidence of stent fractures and associated valve dysfunction (5,9). In contrast to the experience in the US (10), where presenting was not allowed initially, several large European series used presenting from the beginning routinely and showed that stent fractures were not a clinical problem anymore (1,11).
Placement of a pre-stent provides two advantages. In patients with pulmonary stenosis (PS) it contributes to relief of the pressure gradient by widening the RVOT. Pressure relieve allows implantation of a valve at a larger diameter, optimizing the hemodynamic outcome. In patients with PR, with usually wide RVOTs, a pre-stent serves as an anchor for the valved stent and prevents dislocation of the valve during implantation. Additionally, it minimizes the risk for a paravalvular leakage, especially if a covered stent is used for pre-stenting. However, there are no defined protocols and pre-stenting practices vary among investigators.
The Sapien valve received CE label for the pulmonic position in 2010. In analogy to the Melody valve, implantation of a pre-stent was widely adopted. But in contrast to the weaker CP stent of the Melody valve, the frame of the Sapien valve was designed for the aortic position. The cobalt-chromium frame of the valve features a high radial force and ensures that even thick calcified material can be displaced and an eventual obstruction can be relieved adequately. Therefore, in adult patients receiving transcatheter aortic valve implantation (TAVI), the Sapien valve is usually implanted without a pre-stent (7,8). Taking these advantages into consideration, implantation of a Sapien valve without a pre-stent in pulmonic position has been already reported as a case report (12).
Specific risks are associated with the implantation of a pre-stent. Pre stenting tends to lengthen the procedure time, complications related to the placement of a pre-stent like embolization or crushing while advancing the delivery system constitute an additional risk for the patient (11). A longer procedure goes hand in hand with longer fluoroscopy times, inevitably increasing the radiation dose for the patient. Implantation of a pre-stent means more foreign material in the human body creating an additional risk for inflammation. Finally, the unprotected Sapien valve might be entangled in the pre-stent, possibly leading to dislocation of the pre-stent or the tricuspid valve might be damaged during difficult rescue procedures in order to get the valve free for deployment.
Limitations
This is a descriptive and retrospective report. However, procedural safety must be proven in larger studies.
Conclusions
In conclusion, direct implantation of the Sapien XT/S3 in pulmonic position in selected patients yields encouraging results. The procedure is simplified and hence procedural—and fluoroscopy times are reduced. In wide RVOTs it is our feeling, that the implantation of a Sapien valve with the current delivery system is technically easier in absence of a pre-stent in the pulmonary artery.
Acknowledgments
None.
Footnote
Conflicts of Interest: P Ewert is proctor for Medtronic and Edwards. A Eicken is proctor for Medtronic. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All patients provided written informed consent for the procedure and data acquisition was approved by the local ethics committee (No. 281/18 S).
References
- Eicken A, Ewert P, Hager A, et al. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. Eur Heart J 2011;32:1260-5. [Crossref] [PubMed]
- Hager A, Schubert S, Ewert P, et al. Five-year results from a prospective multicentre study of percutaneous pulmonary valve implantation demonstrate sustained removal of significant pulmonary regurgitation, improved right ventricular outflow tract obstruction and improved quality of life. EuroIntervention 2017;12:1715-23. [Crossref] [PubMed]
- Georgiev S, Tanase D, Ewert P, et al. Percutaneous pulmonary valve implantation in patients with dysfunction of a "native" right ventricular outflow tract - Mid-term results. Int J Cardiol 2018;258:31-5. [Crossref] [PubMed]
- Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000;356:1403-5. [Crossref] [PubMed]
- Cabalka AK, Hellenbrand WE, Eicken A, et al. Relationships Among Conduit Type, Pre-Stenting, and Outcomes in Patients Undergoing Transcatheter Pulmonary Valve Replacement in the Prospective North American and European Melody Valve Trials. JACC Cardiovasc Interv 2017;10:1746-59. [Crossref] [PubMed]
- Nordmeyer J, Khambadkone S, Coats L, et al. Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation 2007;115:1392-7. [Crossref] [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Sawaya F, Kappetein AP, Wisser W, et al. Five-year haemodynamic outcomes of the first-generation SAPIEN balloon-expandable transcatheter heart valve. EuroIntervention 2016;12:775-82. [Crossref] [PubMed]
- Nordmeyer J, Lurz P, Khambadkone S, et al. Pre-stenting with a bare metal stent before percutaneous pulmonary valve implantation: acute and 1-year outcomes. Heart 2011;97:118-23. [Crossref] [PubMed]
- Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation 2015;131:1960-70. [Crossref] [PubMed]
- Cools B, Brown S, Budts W, et al. Up to 11 years of experience with the Melody valved stent in the right ventricular outflow tract. EuroIntervention 2018;14:e988-94. [Crossref] [PubMed]
- Ghobrial J, Levi DS, Aboulhosn J. Native Right Ventricular Outflow Tract Transcatheter Pulmonary Valve Replacement Without Pre-Stenting. JACC Cardiovasc Interv 2018;11:e41-4. [Crossref] [PubMed]


