
Integrated use of cardiac MRI and the CardioMEMS™ HF system in PAH: the utility of coincident pressure and volume in RV failure—the NHLBI-VITA trial
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease leading to right-sided heart failure (HF) and death with survival rates of 68% at 3 years (1,2). Goal-oriented treatment strategies must be continually adapted to each patient’s changing status. Evaluation of right ventricular (RV) function and ventricular-vascular coupling (VVC) are fundamental practices in managing and predicting outcome in PAH patients. Evaluating these features on a day-to-day basis is not typically feasible due to the general requirements of invasive testing employing high fidelity instruments. Unfortunately, these measurements typically require special equipment and invasive procedures to simultaneously measure intracardiac pressure and volume. However, the CardioMEMS™ HF System (Abbott Laboratories, Illinois) after permanent implantation in the pulmonary artery (PA) during right heart catheterization (RHC), can be used to remotely monitor on a frequent basis PA pressure (PAP) in HF and PH patients (3-6). If desired, data can be collected daily or even hourly using the home-based sensor and transmission system (7). We tested the safety and feasibility of using this system in combination with cardiac MRI (cMR) to obtain coincident pressure and right heart cMR volumetric data in patients with PAH as part of an NHLBI sponsored trial (HHSN268201400008C). An advantageous feature of cMR is that it provides a high level of accuracy for a wide variety of dimensional, volumetric and flow data. In the short term, cMR and CardioMEMS data can be combined to provide indices of cardiac function not available to either source separately (8). In the longer term, this combined has potential to provide new insights into RV physiology that may prove to be of prognostic value. Following this discovery period, the lessons and insights may be translated for use with additional modalities such as echocardiography and catheterization (9). The safety and responsiveness of acquiring near-simultaneous pressure and volumetric data is demonstrated at: (I) baseline, during (II) nitric oxide inhalation, (III) dobutamine (Dob) infusion and (IV) volumetric loading. We demonstrated for the first time that simultaneous use of CardioMEMS in the magnetic field is feasible and that the corresponding cMR volumetric and PAP data can be acquired safely and interpreted to characterize the underlying physiology of each patient. Herein, we demonstrate the feasibility and safety of near simultaneous acquisition of cMR and CardioMEMS data. This combined technique may have future value as an improved clinical and research tool in prognosticating and studying the underlying pathophysiology of PAH.
Methods
Overview
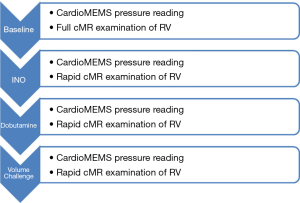
PAH patients with predominantly NYHA class III and IV symptoms and a recent (<30 days) hospitalization for right HF, were enrolled into the NHLBI (VITA) study after giving informed consent for this IRB approved study. The CardioMEMS sensor was implanted into the right PA according to manufacturer instructions during RHC. To obtain measurements from the CardioMEMS system, the patient lies supine on a transmit/receive coil about 50 cm diameter (tuned to each specific CardioMEMS device). When activated, the coil transmits RF energy towards the device, which powers circuitry in the implanted device, which subsequently re-transmits RF energy back to the coil for signal reception. Encoded in the retransmitted signal is the time resolved PA pressure information, sampled at 8ms intervals. One month post implantation, to allow complete stabilization of the implant, patients underwent RV/PA evaluation using a cMRI non-contrast protocol to measure RV volumes and dimensions along with quantitative blood flow in the main PA and near simultaneous acquisition of PA pressures using the CardioMEMS device. The examination was performed at baseline conditions over a period of approximately 30 minutes. Following this a rapid examination protocol was conducted to measure volumetric and pressures conditions under three challenge states: inhaled nitric oxide (INO), Dob, and volumetric challenge (Figure 1). The time-resolved CardioMEMS pressure data was summarized to yield the PA end-diastolic pressure (EDP), RV end systolic pressure (ESP), mean pulmonary artery pressure (mPAP) and heart rate (HR). Combining the near-coincident cMRI-derived volumetric measurements and the CardioMEMS-derived pressure measurements allowed the calculation of maximal RV myocardial elastance (Emax), maximal PA elastance (Empa), ventricular vascular coupling (VVC) ratio and cardiac index (CI), using the following equations (10,11):
Emax = (ESP – EDP)/ESV [1]
Empa = (ESP – EDP)/SV [2]
VVC = ESV/SV [3]
CI = (HR × SV)/BSA [4]
To accomplish the imaging and pressure measurements during a single scan session a series of protocols were developed as outlined below.
Protocol: baseline cMR examination
Prior to formalizing the cMR imaging protocol, we established that the phased-array cMR coils could interfere with operation of the CardioMEMS transmit/receive system. As it was deemed too disruptive to position and remove the cMR phased-array coils to perform each pressure measurement in concert with the cMR examination, all cMR imaging was performed using the body transmit-receive coil system. Prior to development of phased array coils, examinations were routinely conducted with the body coil, which yielded images of sufficient quality for this analysis (12). The baseline protocol was:
- Position patient on cMR table without phase-array coils (due to the interaction with the CardioMEMS sensor), landmark at four inches above the zyphoid, place EKG leads and establish triggering signal;
- Perform three sets of orthogonal scout scans under breath-hold (BH) conditions;
- Perform two chamber long axis cine examination (BH);
- Perform four chamber long axis cine examination (BH);
- Perform multiple RV short axis views planned from the four and two chamber images, with contiguous coverage from base to apex (separate BH for each slice);
- Perform orthogonal cross-sectional view of the main pulmonary artery ~1 cm above the pulmonic valve using velocity encoded cine images of cardiac outflow during free breathing.
Protocol: establishment of stress conditions
The protocol to establish each of the stress conditions was as follows:
- INO: with the patient continuing to lay supine on the cMR scanning table, the table was slid out of the scanner. A nasal cannula was inserted into the patient’s nostrils and nitric oxide at 20 ppm/L/min was inhaled over a 10 minute period. After 10 minutes had elapsed, the CardioMEMS PA pressure readings were obtained. Following this the patient was re-positioned in the scanner and the reduced rapid cMR protocol performed;
- Dob infusion: with the patient continuing to lay supine on the MRI scanning table, the table was slid out of the cMR scanner and the nitric oxide inhalation terminated. A Dob infusion pump was connected to a venous port in the patient’s right arm. Infusion of Dob was initiated at 5 µg/kg/min for three minutes. During the Dob infusion, the patient’s heart rate and blood pressure were monitored. After three minutes, the Dob dose was increased to 10 µg/kg/min and after a further three minutes the dose was increased to 20 µg/kg/min. When the dose of 20 had been established for three minutes, the CardioMEMS PA pressure reading was initiated. Following this the patient was re-positioned in the scanner and the reduced rapid imaging protocol was conducted;
- Volume challenge (Vol): with the patient continuing to lay prone on the cMR scanning table, the table was removed from the scanner and the Dob infusion terminated. A 1,000 mL bag of saline fluid was connected to a venous port in the patient’s right arm. The rate of saline solution infusion was adjusted such that 500 mL of saline was administered rapidly over at least a 20 minute interval to allow the effects of Dob to dissipate. When the amount of saline approached 500 mL, the rate was reduced to keep vein open (KVO) and a CardioMEMS reading was taken. Following this the patient was re-positioned in the scanner and the reduced rapid protocol was conducted.
Protocol: rapid stress cMR
The reduced cMR stress protocol was:
- Perform four chamber long axis view cine examination;
- Perform orthogonal cross-sectional view of main pulmonary artery using velocity encoded cine images of cardiac outflow.
Protocol: cMR scan sequences
The following acquisition sequences and parameters were used during cMR imaging:
- Cine scans: steady state free precession (SSFP) scanning, matrix 256×192, 50 ms heart-phase interval over the cardiac cycle, slice thickness 8 mm, TR/TE/flip angle 3.7/1.2/40, scan time 10–20 s (depending on views per segment and heart rate), all data acquired during a breath-hold, field of view 30–40 cm, depending on patient dimensions;
- Velocity scans: gradient recalled echo (GRE) matrix 256×192, 50 ms per cardiac phase, TR/TE/flip angle, 7/4/20, field of view 30–40 cm, depending on patient dimensions, velocity encoded range 1.5 m/s applied in a through plane manner. To reduce motion artifacts, two signals were averaged during the free breathing scan.
Protocol: cMR measurements
For the baseline cMR measurement, the multiple-slice short axis data set was used to measure the RV volumes (13,14). The endocardial boundaries of the RV were identified on each slice of the series and contoured using standard cMR analysis software (Medis, Leiden, The Netherlands). The end-systolic and end-diastolic frames were identified and the end-diastolic and end-systolic volumes measured (15). These values were used to calculate the ejection fraction at baseline. The RV stroke volume (SV) was measured as the flow volume through the main PA assessed by the cMR phase velocity scan (16). The boundary of the main PA was drawn (Medis Qflow, Leiden, The Netherlands) in the flow images and the flow volume calculated.
For safety reasons, the stressor conditions were held for the shortest possible time duration while data were acquired. Consequently, the reduced rapid cMR protocol did not acquire the multi-slice short axis scans required to measure the volume of the RV at end-systole and end-diastole. This necessitated development of an approach to measure the RV EF from the 4-chamber view, which shows the RV in long-axis orientation (Figure 2). Conventionally, the volumes of the LV can be assessed from a single long axis view such as from the horizontal long axis view (e.g., the 4-chamber view) by outlining the endocardial borders at end systole and at end diastole and using a volume of rotation approach (Sandler-Dodge method) (17). This approach assumed that the LV was rotationally symmetric, and this is a restraint that can be relaxed if the perpendicular long axis view of the LV was also acquired (vertical long axis view, or 2 chamber view) and thus data from each view only requires 90° of rotation (18). However, unlike the LV the RV is not rotationally symmetric. Nevertheless, we hypothesized that the Sandler-Dodge approach could be used in a limited manner to allow the EF to be calculated (19). The great difficulty in this approach is recognizing the true endocardial boundary in the presence of papillary muscles and trabeculae (20). Thus, to make the approach suitable for use in the RV it requires a training set for each patient. In our case the multi-slice short axis data set provided the necessary training set at baseline conditions, allowing successful identification of the endocardial boundary in the long axis view. The RV EF was measured in the 4-chamber view for each of the stress conditions. Knowledge of the RV EF from the 4-chamber view and SV from the flow image was used to calculate the end-diastolic volume (EDV) and end systolic volume (ESV) of the RV using the following equations:
EDV = SV/EF [5]
ESV = EDV – SV [6]
Protocol: acquisition of CardioMEMS pressure data
Prior to acquisition of the cMR baseline examination, the patient was instructed to remain still on the cMR table while out of the cMR scanner, but with the table still attached to the scanner. The CardioMEMS transmit-receive coil was slid under the back of the supine patient (outside of the 5 Gauss line). After waiting one minute for the patient to stabilize, the CardioMEMS measurement was initiated. During this time, two sets of dynamic PA pressure measurements were performed for 10 seconds each at a rate of 8ms per time point. The patient was slid into the scanner and the baseline cMR examination performed. In this way, the cMR and CardioMEMS data were acquired in a near-simultaneous manner. These results at baseline were compared with pressure readings taken at the patient’s home under resting conditions prior to the cMR examination and following the cMR examination.
Following acquisition of the baseline cMRI/CardioMEMS evaluation the patient was brought out of the scanner (but remaining on the scanner table) and the first stress condition established. Once established the CardioMEMS transmit-receive coil was slid under the patient’s back and pressure readings taken. After removal of the CardioMEMS coil the patient was advanced into the scanner for performance of the rapid-scan cMR protocol. This procedure was repeated for the remaining two stress conditions of Dob stress and volume challenge. Of note, time was allowed for each patient’s hemodynamics to return to baseline between pharmacologic interventions, but due to the overlap in recovery and establishment of the next stressor condition, return to baseline could not be generally confirmed.
Statistical analysis
Demographic data were summarized as mean and standard deviation or number and percentage. Bland-Altman analysis was used to compare measurements of RVEF by the reduced cMR protocol and the volumetric cMR protocol. Pearson’s correlation r2 was used to compare measurements of heart rate between the first and second readings of CardioMEMS and the cMR measurement. Paired Student’s t-testing was used to compare measured and derived variables between baseline and each stress condition. Analysis of variance with repeated measures was used to compare CardioMEMS pressure readings prior to, during and post cMR. Significance was regarded as a P value <0.05. Data were analyzed using PASW Statistics (version 18.0) software (SPSS Inc., Chicago, IL, USA).
Compliance of ethical statement
Informed consent was obtained from each patient and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The Institutional Research Board of Alleghany General Hospital approved this study: No. 5850, “Vascular Interventions/Innovations and Therapeutic Advances (VITA); A study to Explore the feasibility of Using Combined Modalities to test the Safety of CardioMEMS Device in PAH Patients”.
Results
Patients
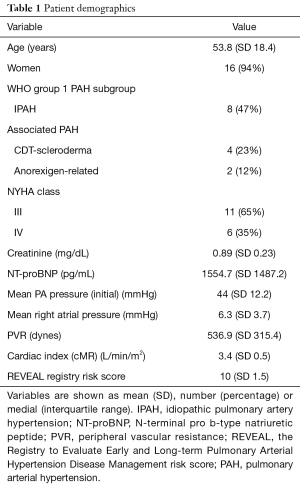
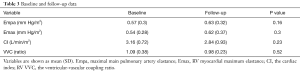
Seventeen PAH patients with predominately NYHA FC III (11, 65%) or IV (6, 35%) symptoms were enrolled. Demographics and hemodynamics at time of implant are noted in Table 1. The mean time between consent and implantation was 15±26 days to ensure clinical stability in treatment-naïve patients post hospital discharge. All were successfully imaged at baseline (1 month post implant), with 12 (71%) patients returning to complete the cMR follow-up at 4 months post (failure to complete follow-up was due to worsening medical issues not related to cMR). Demographics in Table 1 derive from cMR volumetric, CardioMEMS pressures and other sources.
Full table
Safety and image quality
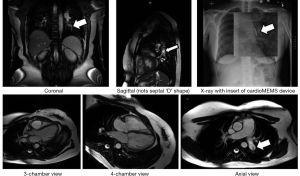
All 29 cMR examinations were completed without incident or patient safety issues. Further, the presence of the CardioMEMS device was not discernible in the images and did not result in any extended paramagnetic artifact or a visual field disturbance using the body coil. Under careful interrogation by an author well-versed in cMR, on review of the images at baseline and follow-up, no paramagnetic artifact or a visual field disturbance was noted (Figure 2).
Physiologic parameters
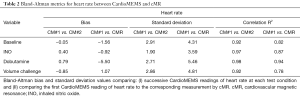
During the one month post implant time point, at each physiologic test, two CardioMEMS readings were taken for each corresponding cMR set of measurements. The only parameter that was common to both CardioMEMS and cMR was the heart rate. The results of the Bland-Altman analysis between the two CardioMEMS readings of heart rate were compared to each other and the first CardioMEMS reading of heart rate compared to the corresponding cMR reading, Table 2. The correlation r2 values ranged from 0.92 to 0.98, for the two CardioMEMS readings and from 0.78 to 0.94 for CardioMEMS to cMR measurements, indicating excellent reproducibility. For the baseline and INO conditions the cMR-CardioMEMS bias terms are very low, while the bias increases for the Dob challenge (reflecting higher variation in heart rate) and the fidelity of the measurements returns for the volume overload challenge conditions which were the last challenge performed. To establish that the CardioMEMS pressure data was not affected by the cMR environment, the ANOVA analysis of pressures prior to (7±1 days), during and following (7±1 days) cMR was performed separately for systolic, diastolic and mean pressure readings and showed no statistical differences (P=0.35, 0.50 and 0.43, respectively).
Full table
RV EF measurements
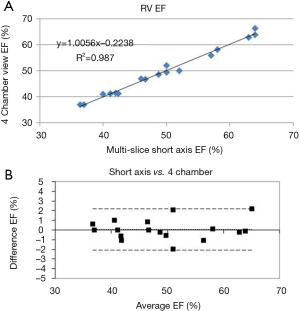
The RV EF was estimated from the four-chamber view by using the baseline data as a training guide to distinguish between papillary muscle and trabeculae for each patient. Results of the Sandler-Dodge area-length approach applied to the baseline data are shown in Figure 3 where the correlation r2 is 0.99 and the Bland-Altman bias term is 0.05% with two standard deviations being 2.1% (21). This knowledge was then applied to the stressor 4-chamber views to better and more quickly estimate the EF. From knowledge of the EF and the RV output from the phase velocity scan of the main PA we were able to calculate the end-diastolic and end-systolic RV volumes.
Combined measurements
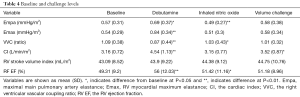
For all patients, the cMR and CardioMEMS data were obtained in a time resolved manner throughout the cardiac cycle (~40–50 ms temporal resolution for cMR cine image and 8ms temporal resolution for CardioMEMS for a ten second period). Here we were able to use volumetric and combined volumes and pressures at end-systole and end-diastole to obtain estimates of Emax, Empa, Cardiac Index and the VVC ratio. Table 3 shows results of key physiologic variables at baseline for 1 month vs. the four month follow-up visit. The average value of Empa, Emax, CI and VVC ratio measured at both time points show no significant difference. Table 4 shows representative volumetric and volumetric-pressure derived variables at baseline and at each of the stress conditions. In Table 4, the variables that differ from baseline for each stress condition are indicated by * for P<0.05 and by ** for P<0.01.
Full table
Full table
Discussion
We have successfully demonstrated the safety and feasibility of using the CardioMEMS device in the MRI environment. The importance of this finding lies in the ability to exploit the integration of near simultaneous hemodynamic and volumetric data for quantitation of such metrics as Emax and Empa. These prognostic variables, if further validated serially, could be used to advance the field of risk stratification in this vulnerable population. Herein, we also demonstrate that clinically routine and contemporary relevant information can be obtained with near simultaneous acquisition of a truncated cMR examination for RV volumes and a CardioMEMS evaluation of PAP. Our choice to use the body coil for transmission was driven both by the requirement for the patient to remain on the cMR table during CardioMEMS interrogation, and to minimize the risk of patient movement (preventing re-scouting of the patient) as would have been required if surface coils had to be removed and repositioned for each CardioMEMS pressure reading. This choice resulted in a signal reception that did not interfere with CardioMEMS signal reception coil. While image quality is slightly lower than that achieved using phased array surface coils it was nevertheless sufficient for accurate volumetric assessment. Similarly, in cases where cMR is required but interrogation of the CardioMEMS device is not needed, we also established that phase-array surface coils can be used without any compromise due to the presence of the CardioMEMS device. This is a critical concept as cMR is being used with increased frequency to follow RV function in response to therapy in many PAH Centers globally including ours.
In order to maximize patient comfort and to allow multiple stress conditions to be performed, a truncated cMR protocol was utilized to limit time under each stressor condition. In this case, a 4-chamber (horizontal long-axis) view was used to assess the RV EF. While the RV is not rotationally symmetric (as the LV approximates to) the area-length calculation had the correct dimensions for EF and estimated EF well under these highly-guided conditions. We are not proposing that the 4-chamber view is generally acceptable to estimate RV EF, but in this case, where we were able to train the drawing of boundaries on an individual basis, acceptable results were obtained.
We demonstrated the safety and feasibility of near-simultaneous cMR and CardioMEMS and showed that the cMR environment did not systematically influence the CardioMEMS pressure readings. These parameters do not specifically utilize the time-resolved nature of the data (other than at the two key time points of end systole and end diastole). Additional studies are planned to investigate the relationships between the synchronized cMR and CardioMEMS time resolved pressure, blood flow and cardiovascular volume data and clinical outcome to enhance the already useful hemodynamic assessments derived from the CardioMEMS device in monitoring patients with PAH (22) and progressing towards predicting outcome (23).
Importantly, we are not proposing that this approach would supplant traditional approaches of obtaining VVC, Emax and other unique RV metrics, but we advance the notion that cMR when interleaved with CardioMEMS offers a unique clinical opportunity to optimize patient evaluations by efficiently reducing the downstream invasive nature of determining such characteristics and by increasing the number of virtual touch points with a patient by optimizing the information obtained by the daily recordings from the CardioMEMS device. Since the ongoing status of RV health is at the core of prediction modeling in PH, the combination of intermittent cMR imaging and daily RV metric evaluation form the CardioMEMS device offers a unique monitoring algorithm designed specifically for the PH patient. Prospective validation of this combined approach is needed in PAH to determine its ultimate role amongst other risk guided treatment algorithms (24-27) before widespread utilization of this approach is ready for everyday clinical use.
Limitations
A number of limitations are noted. The methods and results presented here are limited to demonstrating the feasibility of combining cMR and CardioMEMS during stress conditions. To our knowledge, we are the only center to routinely perform simultaneous cMR and CardioMEMS interrogations, and the general approach may find greater use in clinical research applications, in part, the goals of our ongoing NHLBI Trial. This study was not designed to demonstrate the utility of provocative testing but to show that it could be performed safely in the cMR scanner. The ‘stress’ testing was performed in a fashion to reasonably permit ‘return to baseline’, but demonstration of this was not always feasible given the overlap of recover from one stress and establishment of a second stress.
Conclusions
Non-invasive assessment of hemodynamic and physiologic conditions via cardiac MRI is safe and efficacious when integrating a novel, implantable hemodynamic monitor, CardioMEMS. Utilizing this concept, we show under resting and stress conditions that contemporary physiologic change in cardiac and arterial response within the RV and PA can readily be assessed, paving the way for more sophisticated and integrated approaches.
Acknowledgments
Funding: Funding for this project was provided through an NIHLB VITA grant (HHSN268201400008C) with salary support provided for all authors.
Footnote
Conflicts of Interest: RL Benza has a consulting relationship with Abbott Laboratories. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Research Board of Alleghany General Hospital (No. 5850) and written informed consent was obtained from all patients.
References
- Fakhri AA, Hughes-Doichev RA, Biederman RW. Imaging in the evaluation of pulmonary artery hemodynamics and right ventricular structure and function. Heart Fail Clin 2012;8:353-72. [Crossref] [PubMed]
- Davey R, Raina A. Hemodynamic monitoring in heart failure and pulmonary hypertension: From analog tracings to the digital age. World J Transplant 2016;6:542-7. [Crossref] [PubMed]
- Loh JH, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System. J Am Coll Cardiol 2013;61:1571-6. [Crossref] [PubMed]
- Benza RL, Raina A, Abraham WT, et al. Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant 2015;34:329-37. [Crossref] [PubMed]
- Mehmood M, Agarwal R, Raina A, et al. Hemodynamic response to treatment of iron deficiency anemia in pulmonary arterial hypertension: longitudinal insights from an implantable hemodynamic monitor. Pulm Circ 2016;6:616-8. [Crossref] [PubMed]
- Airhart S, Verlinden N, Badie N, et al. Use of an implantable wireless pulmonary pressure monitor during transition of therapy in pulmonary arterial hypertension. J Heart Lung Transplant 2019;38:227-30. [Crossref] [PubMed]
- Bourge RC, Abraham WT, Adamson PB, et al. Randomized Controlled Trial of an Implantable Continuous Hemodynamic Monitor in Patients With Advanced Heart Failure The COMPASS-HF Study. J Am Coll Cardiol 2008;51:1073-9. [Crossref] [PubMed]
- Benza RL, Doyle M, Lasorda D, et al. Monitoring Pulmonary Arterial Hypertension Using an Implantable Hemodynamic Sensor. Chest 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Mehmood M, Biederman RWW, Markert RJ, et al. Right Heart Function in Critically Ill Patients at Risk for Acute Right Heart Failure: A Description of Right Ventricular-Pulmonary Arterial Coupling, Ejection Fraction and Pulmonary Artery Pulsatility Index. Heart Lung Circ 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Sanz J, Garcıa-Alvarez A, Fernandez-Friera L, et al. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart 2012;98:238-43. [Crossref] [PubMed]
- O’Rourke MF, Yaginuma T, Avolio AP. Physiological and pathophysiological implications of ventricular/vascular coupling. Ann Biomed Eng 1984;12:119-34. [Crossref] [PubMed]
- Asher KA, Bangerter NK, Watkins RF, et al. Radiofrequency Coils for Musculoskeletal MRI. Top Magn Reson Imaging 2010;21:315-23. [Crossref] [PubMed]
- Benza R, Biederman R, Murali S, et al. Role of cardiac magnetic resonance imaging in the management of patients with pulmonary arterial hypertension. J Am Coll Cardiol 2008;52:1683-92. [Crossref] [PubMed]
- Biederman RW. Cardiovascular magnetic resonance imaging as applied to patients with pulmonary arterialhypertension. Int J Clin Pract Suppl 2009.20-35. [Crossref] [PubMed]
- Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson 2013;15:35. [Crossref] [PubMed]
- Kondo C, Caputo GR, Semelka R, et al. Right and left ventricular stroke volume measurements with velocity-encoded cine MR imaging: in vitro and in vivo validation. AJR Am J Roentgenol 1991;157:9-16. [Crossref] [PubMed]
- Sandler H, Dodge HT. The use of single plane angiocardiograms for the calculation of left ventricular volume in man. Am Heart J 1968;75:325. [Crossref] [PubMed]
- Hundley WG, Bluemke D, Bogaert JG, et al. Society for Cardiovascular Magnetic Resonance guidelines for reporting cardiovascular magnetic resonance examinations. J Cardiovasc Magn Reson 2009;11:5. [Crossref] [PubMed]
- Goshtasby A, Turner DA. Segmentation of cardiac cine MR images for extraction of right and left ventricular chambers. IEEE Trans Med Imaging 1995;14:56-64. [Crossref] [PubMed]
- Chuang ML, Gona P, Hautvast GLTF, et al. Left Ventricular Trabeculae and Papillary Muscles: Correlation With Clinical and Cardiac Characteristics and Impact on Cardiovascular Magnetic Resonance Measures of Left Ventricular Anatomy and Function. JACC Cardiovasc Imaging 2012;5:1115-23. [Crossref] [PubMed]
- Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. Statistician 1983;32:307-17. [Crossref]
- Raina A, Abraham WT, Adamson PB, et al. Limitations of right heart catheterization in the diagnosis and risk stratification of patients with pulmonary hypertension related to left heart disease: Insights from a wireless pulmonary artery pressure monitoring system. J Heart Lung Transplant 2015;34:438-47. [Crossref] [PubMed]
- Vanderpool RR, Pinsky MR, Naeije R, et al. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart 2015;101:37-43. [Crossref] [PubMed]
- Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122:164-72. [Crossref] [PubMed]
- Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012;141:354-62. [Crossref] [PubMed]
- Kylhammar D, Kjellstrom B, Hjalmarsson C, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2018;39:4175-81. [Crossref] [PubMed]
- Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017. [Crossref] [PubMed]


