Utility of balloon assisted technique in trans catheter closure of very large (≥35 mm) atrial septal defects
IntroductionOther Section
Trans catheter closure (TCC) for atrial septal defects (ASDs) has been in vogue for more than two decades (1). ASD is considered as large when the long axis measurement is ≥25 mm (2-4). In clinical practice, we often encounter defects, which are very large (≥35 mm). The treatment decision for these patients is unclear. Traditional management includes surgical closure, but morbidity of the open-heart procedure, presence of scar and longer hospital stay makes surgical closure unattractive to many patients. The published studies show ASD size more than 25 mm is a risk factor for unsuccessful device deployment (3,5). Size more than 38 mm is generally considered as a contra indication to TCC (6). But the international registry data shows feasibility of deploying 40 mm Amplatzer device in TCC of large defects up to 39 mm (6). Modified device deployment techniques have been suggested to facilitate device alignment and increase the success rates of TCC (3,4,6).
We hypothesized that the balloon-assisted technique (BAT) would increase success rates for TCC of very large ASDs compared to conventional techniques (CT). We retrospectively studied the utility of BAT as against the CT in TCC of very large ASDs measuring ≥35 mm over the past 5-year period (January 2008 to December 2012) at our institute and to find out the association of different morphological features of the ASD which could negatively affect device alignment to TCC outcomes.
Design and methodsOther Section
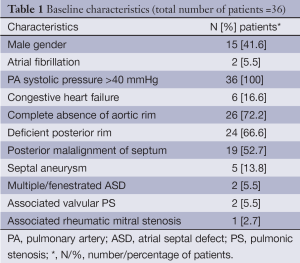
The study was conducted in a tertiary care referral hospital experienced in the TCC of ASD. All patients with large ASD measuring ≥35 mm who were referred for TCC were initially evaluated with trans thoracic echocardiography (TTE) followed by trans esophageal echocardiogram (TEE). As surgery is the conventional treatment for these large ASD’s, we included patients after a meticulous echo imaging and who preferred a non-surgical mode of therapy after counseling. Inclusion criteria were very large ostium secundum ASD with any one of the measurements in anterior-posterior or superior-inferior plane measuring ≥35 mm. Secundum ASD is often oval in shape and measurements vary in different planes. Deficient rim was defined as ≤5 mm and adequate rim as ≥5 mm. Morphological features which were considered challenging for device alignment and successful TCC outcome included complete absence of aortic rim, floppy or absent posterior rim, septal aneurysm and posterior mal-alignment of the septum. Posterior malalignment was defined as change in the posterior septal plane on TEE sweep from 0 to 90 degrees. These features were recorded separately in patients who underwent TCC of very large ASD and their influence on outcomes with regard to BAT and CT were compared. Associated valvular lesions like mitral/pulmonic stenosis were included after successful balloon valvuloplasty. We excluded patients who had pulmonary artery hypertension with catheterization hemodynamics suggestive of inoperability, complete absence of inferior and or inferior vena caval rim and size ≥44 mm as the largest occluder available to us from the manufactures was 44 mm. All Patients were counseled regarding the surgical and catheter options available and the nature of intervention. Informed consent was obtained from patients for the procedure before the intervention and for participation in the study at their follow up visits. The study was started after approval from the institute ethics committee. Data collection was retrospective and included detailed TEE assessment for morphological features of ASD in each patient and cardiac catheterization findings. Follow up data included clinical and TTE studies.
Meticulous TEE imaging of all the very large defects were done with 0-120 degrees sweeping in addition to the standard imaging angles at 0, 45 and 90 degrees. TEE was done in presence of the interventionalist in view of the complexity of the defects. All procedures were done under general anesthesia and with TEE guidance. Heparin was administered at 100 U/kg of with maintenance of activated clotting time between 250-300 seconds. Balloon sizing of the defect was done only in some cases where TEE size assessment was uncertain.
The technique of device closure (CT vs. BAT) was at operator’s discretion, however CT was initially used in majority of cases and if fails, BAT was used. The CT included (I) device deployment from left atrial (LA) chamber (II) upper pulmonary veins (III) the pulmonary vein deployment technique from either of these upper veins. As the learning curve with BAT improved during the study period, some operators directly adopted BAT without trying CT. In some cases, directly BAT was used. For the BAT, the sizing balloon (filled with premeasured quantities of diluted contrast) was kept across the ASD through a contralateral femoral vein access. Once the device is set to be delivered outside the sheath in the left atrium, the balloon was inflated to occlude the defect. This maneuver was done under fluoroscopic and TEE guidance. The device is then delivered from either the left upper/right upper pulmonary vein and the balloon over the wire placed either in the ipsilateral/contralateral pulmonary veins or in the body of left atrium if balloon stability is satisfactory. With the inflated balloon in situ across the defect, the LA disc is deployed and the whole assembly is pulled back against the septum. The balloon supports and prevents prolapse of the LA disc in to right atrium (RA), thereby allowing the RA disc to be deployed over the right side of the inter atrial septum. The device would typically assume an hourglass shape initially. After checking the position of the discs on their respective sides of the septum by TEE, the operator deflates the balloon slowly. With the slow deflation of the balloon, the waist expands and the discs would seem to change the alignment with increasing separation superiorly and decreasing separation inferiorly (better visualized in the left anterior oblique fluoroscopic view). The device position and presence of residual shunt is verified by TEE and once found satisfactory, the balloon is slowly withdrawn in to RA after ensuring complete deflation under fluoroscopic guidance. Often a counter clockwise torque is given to the balloon catheter to facilitate withdrawal. Once the balloon is completely out of the septum, the wire was gently removed and the device position was reconfirmed. If unsatisfactory, the procedure is repeated either with the same device or with upsizing if there was significant residual shunt or rims are not caught well in TEE. Once the device position was satisfactory by TEE and fluoroscopy, the stability of the device further ensured by gentle wiggle before it was released. Successful closure was defined as stable device position after deployment with no or trivial shunt.
The Cocoon (Vascular Innovations) and the Lifetech (Lifetech) ASD occluders were used and the sizing balloons from these manufacture measuring 34 mm for the BAT. This balloon is a 0.035 guide wire compatible double lumen catheter with radio opaque marker bands .The balloon length is 55 mm with maximum inflation volume of 90 mL and can be inflated up to 44 mm without loosing its shape or quality. These occluder devices are similar to the Amplatzer ASD occluder design. The over the wire balloon was kept in LA with wire positioned in right or left upper pulmonary veins or in the left atrium as long as the balloon was stable across the defect.
Follow up
All patients underwent a TTE 24 hours after the procedure to verify the position of the occluding device, residual shunting and resolution of right ventricular systolic pressure. Any complications during and after the intervention were recorded. Patients were on monthly follow up in the first year after intervention and then on yearly basis with TTE done at each visit.
Statistical analysis
Data were analyzed by using both descriptive and inferential statistics. The distribution of continuous data were assessed by using Kolmogrov Smirnov test and accordingly expressed as mean with standard deviation. The statistical significance of the difference in TEE size of defects in success and failure groups was carried out by using independent Students’ t-test. The comparison of different morphological features of ASD with the success of BAT vs. failure of CT was carried out by Fishers’ exact test. All statistical analysis was carried out at 5% level of significance using SPSS version 19.0.
ResultsOther Section
Immediate outcomes
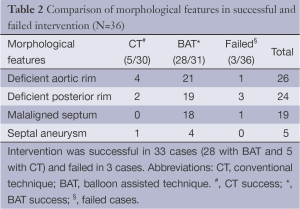
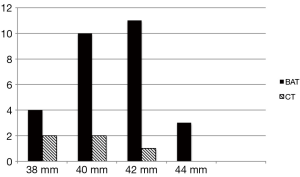
The mean age of the patients was 31.4 (SD 7.9) years, range 17-56 years. Of the total number of 179 patients who were referred for TCC of ASD during the period from January 2008 to December 2012, 36 (21.3%) patients had very large ASD (mean ASD size 36.9 mm). and TCC was attempted. Twenty-three patients with >35 mm ASD underwent surgical patch closure during this period in our institute. Device closure was successful in 33/36 (91.6%) patients with majority of success due to BAT. BAT was attempted in 31/36 patients and was successful in 28 while failed in three cases (success rate of 90.3%). The CT was attempted in 30/36 cases (including pulmonary vein deployment technique in 17 cases) but successful only in 5/36 patients and failed in the majority of 25 patients (success rate of 16.6%). In 23 of these 25 patients with failed CT, BAT was successful. More than 80% of the patients had complete absence of aortic rim and 70% had floppy or absent posterior rims. Posterior mal alignment of the septum was present in more than 50% patients. The baseline characteristics of the patients are given (Table 1). Figure 1 shows the number and size distribution of atrial septal occluders deployed successfully with the balloon assisted and CT. The mean TEE size 37 (SD 1.3) mm and mean device size was 40.8 (SD 1.7) mm in the 28 patients with BAT success was not significantly different than in the CT failure group (P=0.06). The mean trans-esophageal echocardiography (TEE) size with CT failure was 36.2 (SD 1.1) mm while mean device size was 39.6 (SD 1.3) mm. On univariate analysis, posterior mal-alignment of septum was associated with failure of conventional technique (P=0.01). Complete absence of aortic rim (P=0.09), deficient posterior rims (P=0.07) and presence of septal aneurysm (P=0.10) did not differ between failed CT and successful BAT. The morphological features in success/failure groups are given (Table 2).
Full table
Full table
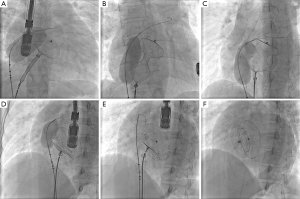
One patient had dextrocardia with a 36 mm ASD as part of Kartagener’s syndrome underwent successful balloon assisted intervention. Balloon sizing of the ASD was done in 15 patients according to the operator’s discretion. All patients who had septal aneurysm (five patients) and fenestrated ASD (two patients) had balloon sizing prior to TCC. In cases where balloon sizing was done, we found a good correlation between balloon sizing by stop flow echo and balloon waist measurement by fluoroscopy with a mean difference of 1.3 (SD 0.3) mm. We did not observe any specific complications related to BAT like rupture of the septum or enlargement of the defect. Figure 2A-F and Figure 3A-F shows sequential TEE and fluoroscopic images of successful balloon assisted 42 mm device closure in a patient with 38.5 mm ASD with deficient posterior rim.

BAT failure
BAT failed in three patients. In two patients having defects larger than 40 mm in size, the device could not continue in the stable position after balloon deflation and withdrawal. The procedure was abandoned and the patients were referred for elective surgical closure. In one patient in whom BAT was attempted the device embolised. The measured size was 35.6 mm and the 38 mm device embolised in to left atrium after deployment. The device was successfully snared out percutaneously. Device was not upsized as the operator felt combined deficiency of aortic and posterior rims would not support a larger device. The patient underwent surgical closure after one week.
Complications
There was no procedure related mortality. Coronary air embolism and transient ST elevation occurred in two patients during procedure without any form of hemodynamic compromise. There was no late embolization of the device. One patient developed new onset pericardial effusion albeit mild, at one-month follow up. It was a cause of concern; the patient was admitted and evaluated for early onset erosion. No findings of erosion were seen in TEE and computed tomography and the effusion subsided spontaneously. There was no worsening of AV valve regurgitation. Two patients with mild to moderate mitral regurgitation at baseline, continued to remain same post procedure. No worsening of tricuspid regurgitation was noted. There was no occurrence of aortic regurgitation.
Follow up outcomes
The mean follow up period for the study is 3.2 (SD 1.7) years, range 8 months-5 years. All patients except one had decreased in right ventricular systolic pressures below 40 mmHg. Six patients with heart failure had resolution of failure symptoms. One patient had small additional ASD, which remained patent after intervention. Residual trivial shunt persisted in one patient at one-year follow up where as in another it disappeared at six months. All other patients had 100% occlusion rates. No new arrhythmias or stroke were reported. All of the patients are on echocardiographic follow up and none of them have any signs of erosion or progressive mitral regurgitation.
DiscussionOther Section
This retrospective study showed 90% success rates with BAT in TCC of very large ASD. Many cases where CT (including pulmonary vein deployment) failed, the device deployment was successfully carried out with BAT. We had high prevalence of complex morphological features like deficient posterior rims (70%) and posterior mal alignment of the septum (50%). BAT had higher success rates despite the fact that the mean ASD size between BAT success and CT failure was not significant. No patient required urgent surgical intervention. We did not have any difficulty in occluding the defect with this highly compliant balloon and open the disk in LA. The disk would obviously deformed significantly, but as we deflate the balloon, the disk assume its shape (The balloon is always inflated to the pre measured TEE size). During BAT, the balloon was not inflated beyond TEE size to stretch the septum as it is done during balloon stretched diameter (BSD) measurement during balloon sizing. Balloon sizing for BSD was done only if TEE sizing is ambiguous.
Long-term follow up was good with no reporting of erosion and residual shunt. The study patients had meticulous TEE imaging before hand in the presence of the interventionalist. ASD size of ≥44 mm was excluded, as we did not have access to occluders above 44 mm. Extreme cases with completely deficient IVC rims were not attempted as the authors had little experience in TCC of such defects.
The mean defect size was significantly larger than the previously published series (3-6). BAT with sizing balloon was earlier published by Fraisse et al. (3) in unselected patients including children. BAT for very large ASD was proposed by Dalvi et al. (4) using circular balloon (Numed). Our study had many differences to the technique proposed by Dalvi. We have used the sizing balloon supplied along with the device loading accessories. We selected randomly both left and right pulmonary veins or even the left atrium for balloon wire position. In many cases we have kept both balloon and sheath on the same side as we encountered balloon stability issues on bilateral approach. We did not had any difference in outcomes with respect to ipsilateral or contra lateral placement of the balloon. The mean ASD size was higher than Dalvi series (36.3 vs. 33.3 mm), mean follow up duration was longer (3.2 years vs. 11.5 weeks) and occluders used were different (Cocoon and Lifetech vs. Amplatzer).
In the setting of a large ASD with complex anatomical features, often the rotatory torque transmitted in a device-loaded sheath is very slow and maintaining the posterior orientation of LA disc is difficult. Often the LA disc tilts tangentially across the defect and prolapse back in to the RA (4). The pulmonary vein deployment technique may become handy in such a scenario as the larger device and relatively smaller LA would favors stretching of the device across the LA and ASD in to the RA (5,6). But we observed that this technique also fails in large ASD with malaligned septum. The crux of success lies in proper device alignment with respect to the defect and surrounding structures. The balloon plays a crucial role in controlling the disc movement and alignment of the device (4). The inflated balloon helps in predictable alignment in a malaligned septum by allowing the LA disc to remain expanded over the left side of the septum while the RA disc fans out over the right side of the septum. This essentially helps the device to “stent” the defect. As the balloon is deflated and withdrawn out of the septum, we observe that the discs on either side tend to re-align to seal the defect.
The success of complex ASD closure mainly lies on the proper imaging techniques (5-12). Complex anatomical substrates like extreme mal alignment with sinusoidal septum, aneurysm, and fenestrated defects require careful delineation before planning intervention (8). TEE may be suboptimal in septal aneurysms and fenestrations/tissue tags straddling ASD (9,10). Balloon sizing was useful in cases where TEE imaging was suboptimal. Balloon sizing was not done uniformly in all patients, as some of the operators did not believe in its utility for sizing besides TEE.
Lack of proper aortic rim is often granted for success, but we found that complete absence of retro aortic tissue is often challenging in large defects when posterior rim also is deficient, because almost always the bigger LA disc flips tangentially across the defect. We did not use three-dimensional TEE (3DTEE), which is becoming increasingly popular in understanding complex septal morphology in TCC of ASD (10,11). Rigatelli et al. showed intracardiac echocardiography guided complex secundum ASD TCC is safe and effective and gives excellent long-term results (12). The authors do not have experience with cardiac computed tomography or cardiac MRI for feasibility for TCC of ASD.
Late device erosion has been reported and is a source of concern (13,14), but we did not find any occurrence of erosion in this series although it remains a concern for us because we had large devices in challenging rims. We are meticulously following up all the patients with echocardiography.
Limitations
The major limitation of this study is its retrospective design. There was no uniformity in the sequence in which different techniques are used for TCC of ASD. Some patients had only BAT, which would have affected the outcomes. The study size also is small in identifying different morphological features associated with CT failure where BAT could be useful. Except for posterior malalignment of the septum, none of the other features could prove statistical significance.
ConclusionsOther Section
Balloon assisted device closure of very large complex ASD had 90% success rate. BAT helps in controlled delivery and device alignment in very large ASD with posterior mal alignment of the septum and is often helpful in cases when CT fails. We did not experience any complications related to BAT.
AcknowledgementsOther Section
Disclosure: The authors declare no conflict of interest.
ReferencesOther Section
- Butera G, Romagnoli E, Carminati M, et al. Treatment of isolated secundum atrial septal defects: impact of age and defect morphology in 1,013 consecutive patients. Am Heart J 2008;156:706-12. [PubMed]
- Rao PS, Sideris EB, Hausdorf G, et al. International experience with secundum atrial septal defect occlusion by the buttoned device. Am Heart J 1994;128:1022-35. [PubMed]
- Kammache I, Mancini J, Ovaert C, et al. Feasibility of transcatheter closure in unselected patients with secundum atrial septal defect, using Amplatzer devices and a modified sizing balloon technique. Catheter Cardiovasc Interv 2011;78:665-74. [PubMed]
- Dalvi BV, Pinto RJ, Gupta A. New technique for device closure of large atrial septal defects. Catheter Cardiovasc Interv 2005;64:102-7. [PubMed]
- Guan Z, Qin Y, Zhao X, et al. Transcatheter closure of large atrial septal defects in 18 patients. Clin Cardiol 2008;31:24-7. [PubMed]
- Lopez K, Dalvi BV, Balzer D, et al. Transcatheter closure of large secundum atrial septal defects using the 40 mm Amplatzer septal occluder: results of an international registry. Catheter Cardiovasc Interv 2005;66:580-4. [PubMed]
- Varma C, Benson LN, Silversides C, et al. Outcomes and alternative techniques for device closure of the large secundum atrial septal defect. Catheter Cardiovasc Interv 2004;61:131-9. [PubMed]
- Santoro G, Bigazzi MC, Lacono C, et al. Transcatheter closure of complex atrial septal defects: feasibility and mid-term results. J Cardiovasc Med (Hagerstown) 2006;7:176-81. [PubMed]
- Schwinger ME, Gindea AJ, Freedberg RS, et al. The anatomy of the interatrial septum: a transesophageal echocardiographic study. Am Heart J 1990;119:1401-5. [PubMed]
- Bhaya M, Mutluer FO, Mahan E, et al. Live/real time three-dimensional transesophageal echocardiography in percutaneous closure of atrial septal defects. Echocardiography 2013;30:345-53. [PubMed]
- Vasilyev NV, Martinez JF, Freudenthal FP, et al. Three-dimensional echo and videocardioscopy-guided atrial septal defect closure. Ann Thorac Surg 2006;82:1322-6; discussion 1326 [PubMed]
- Rigatelli G. Five-year follow-up of intracardiac echocardiography-assisted transcatheter closure of complex ostium secundum atrial septal defect. Congenit Heart Dis 2012;7:103-10. [PubMed]
- Crawford GB, Brindis RG, Krucoff MW, et al. Percutaneous atrial septal occluder devices and cardiac erosion: a review of the literature. Catheter Cardiovasc Interv 2012;80:157-67. [PubMed]
- Fu YC, Cao QL, Hijazi ZM. Device closure of large atrial septal defects: technical considerations. J Cardiovasc Med (Hagerstown) 2007;8:30-3. [PubMed]


