A case of a fibroelastoma and patent foramen ovale in a patient with prior stroke
Catheter-based PFO closures are frequently performed at tertiary referral centers with comprehensive echocardiography performed at the referring sites and limited transesophageal or intracardiac echocardiography during the intervention. We describe a case illustrating the importance of comprehensive transesophageal echocardiography (TEE) prior to device implantation.
Case
This is the case of a 55-year-old male who presented to an outside hospital with a non-disabling middle cerebral artery ischemic stroke. Comprehensive stroke work-up did not reveal any causes for strokes with the exception of a patent foramen ovale (PFO) with right-to-left shunting described on TEE. No further abnormalities were reported. Anticoagulation with a vitamin K antagonist had been recommended but was not pursued by the patient because of a high occupational injury risk. He was, therefore, referred to our institution for percutaneous PFO closure.
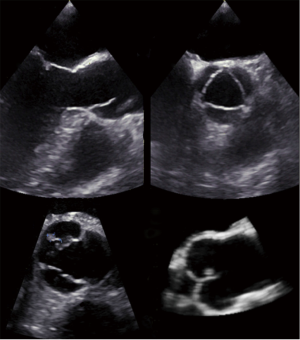
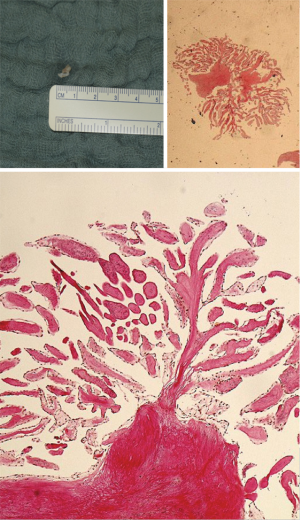
He underwent PFO closure under fluoroscopic and TEE guidance with a 20 mm Premere-Occluder (St. Jude Medical, St. Paul, MN, USA). Focus during intraprocedural TEE imaging was the PFO itself and the interatrial septum. At routine 1-month TEE follow-up, the PFO occluder was well seated and without adherent thrombi. However, a small 5.9 mm × 3.2 mm nodular, mobile echodensity was seen attached via a stalk to the aortic aspect of the non-coronary cusp of the aortic valve prolapsing intermittently with the cardiac cycle into the left ventricular outflow tract (Figure 1 and Video 1). It remained unchanged after one month of anticoagulation with subcutaneous enoxaparin. Of note, thorough review of the TEE performed during PFO closure did reveal the described structure in limited and modified views only. The echocardiographic findings and previous stroke history were highly suspicious for a papillary fibroelastoma and the patient underwent minimally invasive surgical removal via partial upper sternotomy while preserving the structural integrity of the aortic valve. Macroscopic examination revealed a grey-white polyp-shaped 5 mm tissue fragment (Figure 2). Histologically, it displayed the typical features of a papillary fibroelastoma with papillary stroma lined with epithelial cells (Figure 2). After an unremarkable hospital course, the patient was discharged on the fifth postoperative day in excellent condition. Postoperative echocardiography showed an intact and functionally normal aortic valve.
Discussion
Approximately 20% of ischemic strokes are cardio-embolic in nature (1). Hence, TEE is typically part of the routine examinations in stroke patients without otherwise apparent cause. Taking autopsy studies into account, nearly 30% of the general population have a PFO (2). Therefore, a PFO is also a common finding in stroke patients. However, the PFO may be an innocent bystander and other potential causes with stronger stroke association may concomitantly be present. Hence, a thorough interrogation of all structures that may be embolic sources is essential, recognizing that valves that are unremarkable at first impression may harbor subtle but crucial findings that may remain unnoticed unless thorough careful imaging using modified views is routinely performed. Our failure to identify the fibroelastoma emphasizes the importance of comprehensive pre-procedural TEE.
Papillary fibroelastomas, after atrial myxomas, are the second most common primary cardiac tumors (3). The microscopic appearance resembles that of a sea anemone. There is no gender preference and the mean age at presentation is 60 years (3). Tumor sizes at diagnosis vary between 2 and 70 mm (with a mean size of 9 mm) (3,4). More than 80% or papillary fibroelastomas originate from valvular structures, most commonly the aortic (37-45%) and mitral valves (30-36%) (3,4). In 30% of cases, the discovery is incidental, i.e., the patient is asymptomatic and imaging was performed for other reasons (3). Under these circumstances, provided the tumor is small (<1 cm) and non-mobile, conservative management may be reasonable (3,4). However, in the presence of neurologic symptoms suggestive of cerebral or myocardial ischemia or when the tumor is larger (≥1 cm) and mobile, surgical removal should be considered to prevent recurrent embolization (3,4). Tumor excision appears to be curative as, to date, tumor recurrence after successful removal has not been described.
Acknowledgements
Disclosure: Dr. Sievert’s institution has ownership interest in or has received consulting fees, travel expenses or study honoraries from the following companies: Abbott, Access Closure, AGA, Angiomed, Arstasis, Atritech, Atrium, Avinger, Bard, Boston Scientific, Bridgepoint, Cardiac Dimensions, CardioKinetix, CardioMEMS, Coherex, Contego, CSI, EndoCross, EndoTex, Epitek, Evalve, ev3, FlowCardia, Gore, Guidant, Guided Delivery Systems, Inc., InSeal Medical, Lumen Biomedical, HLT, Kensey Nash, Kyoto Medical, Lifetech, Lutonix, Medinol, Medtronic, NDC, NMT, OAS, Occlutech, Osprey, Ovalis, Pathway, PendraCare, Percardia, pfm Medical, Rox Medical, Sadra, Sorin, Spectranetics, SquareOne, Trireme, Trivascular, Velocimed, Veryan.
References
- Sherman DG, Dyken ML Jr, Gent M, et al. Antithrombotic therapy for cerebrovascular disorders. An update. Chest 1995;108:444S-456S. [PubMed]
- Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984;59:17-20. [PubMed]
- Gowda RM, Khan IA, Nair CK, et al. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003;146:404-10. [PubMed]
- Sun JP, Asher CR, Yang XS, et al. Clinical and echocardiographic characteristics of papillary fibroelastomas: a retrospective and prospective study in 162 patients. Circulation 2001;103:2687-93. [PubMed]


