
Cardiovascular risk and D-dimer levels in HIV-infected ART-naïve Africans
IntroductionOther Section
The World Health Organization (WHO) supports the initiation of antiretroviral treatment (ART) for all HIV-infected individuals, independent of their immunologic or clinical status (1). Throughout sub-Saharan Africa (sSA) countries have adopted the “Test-and-Treat” strategy (2), which is expected to contribute to achieving UNAIDS 90-90-90 treatment targets (3). Studies from sSA have highlighted the role of ART in reducing HIV/AIDS-associated morbidity and mortality (4-10), but late presentation to health facilities remains a challenge, maintaining a high risk of death despite availability of effective treatment (4,8,9). With the expansion of ART in Africa cardiovascular disease (CVD) and thrombotic events replaced tuberculous pericarditis and dilated cardiomyopathy as major causes of mortality in the HIV-infected (11-13).
HIV prevalence is over 10% in adult population in Mozambique (14), a country lacking specialized human resources (15), infrastructure for chronic care and NCD surveillance system. The WHO Stepwise Approach to Surveillance (STEPS) surveys of cardiovascular risk factors, revealed high prevalence of hypertension, obesity and alcohol consumption (16-18). In adults 25–64 years old the prevalence of hypertension is 38.9% (19), 3.8% have diabetes and concerning levels of overweight (27.1%) and obesity (10.8%) occur in females (20). We therefore designed a study to assess cardiovascular risk among recently diagnosed HIV-infected ART-naïve patients.
MethodsOther Section
Between 21/05/2015 and 21/12/2015 HIV-positive ART-naïve adult patients diagnosed in WHO classification clinical stage I–II (21) were recruited at a first level referral urban hospital in Maputo, Mozambique. HIV infection was documentated by any licensed ELISA test kit and confirmed by a second method (Western blot). Cardiovascular risk assessment, clinical history and physical examination were performed, including 12-lead electrocardiography and portable transthoracic echocardiography. Left ventricular systolic dysfunction was as defined by left ventricular ejection fraction ≤45% and/or fractional shortening <25%. Venous blood was obtained to measure CD4 count, hemoglobin, glycemia and lipids. A cut-off point of hemoglobin less than 13 (males) and less than 12 (females) was used to define anemia; values lower than 8.0 g/dL defined severe anemia for both sexes. Approximately 1mL of blood obtained from digital puncture was used to determine D-dimers levels using rapid tests (Cobas h 232 PoC handset system - Roche). D-Dimmer was age-adjusted for patients over 50 years [using the formula age (years) × 10 µg/L] (22); values >500 ng/mL were considered abnormal.
All patients initiated ART after the diagnosis as per local management protocols. From May 2018 to August 2018 we assessed the vital status of all participants and the occurrence of major cardiovascular events.
Statistical analysis
Descriptive statistics were computed for demographic, laboratory, echocardiography and HIV-related parameters. Quantitative variables are described with mean (SD) or the median (IQR). To analyze patient’s characteristics by gender, Kruskall Wallis non-parametric test was used to compare medians and t-test to compare means; for qualitative variables Qui-square or Fisher tests were performed. Analyses were done with STATA software and using a 5% significance level.
Ethical considerations
The national bioethics committee approved the study. Informed consent was obtained from all participants.
ResultsOther Section
We recruited 70 patients (41 were females; mean age 37 years, SD 10.7; all of black ethnicity).
Risk factors profile
Three male were smokers. The mean abdominal circumference was 84 cm (SD 10); 21 patients (26.6%) were overweight and 13 (16.7%) obese. The median blood pressure was 119.5/79 mmHg (IQR 107-141/67-83); 20 patients (25.6%) had hypertension. Hypercholesterolemia was present in 20 patients (25.6%)—9 (11.5%) with levels over 7.8 mmol/L—and hypertriglyceridemia in 16 (20.5%). Nineteen patients (20.5%) had hyperglycemia, of which 9 (11.5%) had diabetes. Mean CD4 levels were very low (mean 21.3 cells/mL; SD 10.4). Moderate anemia was found in 28 patients (35.9%) and severe anemia in only one. The median (IQR) hemoglobin level was 11.45 g/dL (9.8–12.8) [12.4 (10.4–13.6) in men vs. 11.1 (9.8–12.2) in women]. There was no difference in levels of blood pressure, glycaemia or cholesterol between males and females; women had lower triglyceride levels (1.3 vs. 1.0 mmol/L, P=0.04) (Table 1).
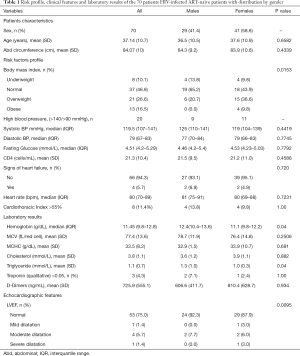
Full table
Cardiac function and biomarkers
Severe impaired left ventricular systolic function was found in 9 patients (shortening fraction below 25%) of which 4 had signs of congestive heart failure. D-dimer levels did not differ between patients with severely impaired left ventricular systolic function [9] and those with normal ventricular function [61], with median 486.67 vs. 548.08 ng/mL, respectively (P=0.93). Three patients had high troponin T levels, but none had clinical or electrocardiographic signs of ischemic heart disease.
Pro-thrombotic markers
High D-dimers levels were found in 44 (62.8%) patients. The mean D-dimers level was 725.9 ng/mL (SD 555.1). No difference was found according to gender—606.6 (SD 411.7) males vs. 810.4 (SD 628.7) females; P=0.934.
Follow-up
On 3-year follow-up 5 patients died and 10 were lost to follow up. The causes of death were: congestive heart failure [2]; pulmonary tuberculosis [1]; unknown [2]. Among the 55 available for follow up, three had had hospitalization due to stroke, heart failure and deep venous thrombosis (Table 2).
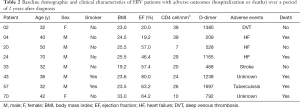
Full table
DiscussionOther Section
This study of recently diagnosed HIV-infected ART-naïve individuals showed high occurrence of overweight, hypertension, hyperlipidemia and hyperglycemia, as well as evidence of pro-coagulant state in a considerable proportion of patients. Impaired left ventricular systolic function was present in a quarter of patients, despite only four having overt heart failure. Our results confirm a double burden of disease in this young population from a low-income setting.
STEPS surveys have been implemented in 42 African countries showing high prevalence of risk factors in some (20). In Mozambique high prevalence of cardiovascular risk factors was found in urban settings (16,18), and a rise in prevalence of hypertension from 33.1% to 38.9% (19) occurred over 10 years. The joint burden of HIV and NCD reduces the quality of life and life expectancy of patients, reducing the impact of the 90-90-90 strategy (3).
Despite HIV-infected people in sSA being at risk of CVD due to aging, exposure to infectious agents, direct consequences of HIV and exposure to specific antiretrovirals (23-30), screening for cardiovascular risk factors or diseases is not systematically performed and NCD-HIV integrated programs are scarce (31). A risk of non-AIDS-related mortality exceeding the risk of AIDS-related mortality has been reported among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3 (32). Our results are in line with estimates pooled from a systematic review of the prevalence of cardiovascular risk factors, which found prevalence of 7.8% (95% CI: 4.3–13.9) for obesity, 21.2% (95% CI: 16.3–27.1) for hypertension, 22.2% (95% CI: 14.7–32.1) for hypercholesterolemia, 27.2% (95% CI: 20.7–34.8) for hypertriglyceridemia and 1.3 to 18% for type-2 diabetes (33).
Our WHO clinical class I-II patients had very low CD4 counts (mean 21.3 cells/mL). A systematic review and meta-analysis of the diagnostic accuracy of this clinical staging system for defining eligibility for ART in sSA revealed that it misses a high proportion of individuals who are ART eligible by CD4 count (34). Thus, because low CD4 count is a strong predictor of thrombotic events in HIV-infected individuals (35,36) and pro-coagulant state favors progression of atherosclerosis, our patients should be considered at higher risk of myocardial infarction, coronary death, ischemic stroke, and peripheral arterial disease (37). The reasons for rise in D-dimers (either related to HIV infection or due to ethnicity factors) need to be clarified in case-control studies matched by ethnic group. Khaleghi and colleagues (38) reported higher levels of D-dimers in African-Americans when compared to other ethnic groups (mean ± SD; men 255±199 vs. 190±182 ng/mL, P<0.001; women, 289±233 vs. 225±195 ng/mL, P<0.001) in North America; ethnicity remained associated with higher D-dimer levels after adjustment for age, conventional risk factors, medication use and lifestyle variables (38). An Italian cohort of 44 HIV non-smoker adult patients on ART and virally suppressed (10 females and 34 males; mean age 48 years, mean CD4+ 674 cells/mL) had only 3 patients with high levels of D-dimers (39), despite a lower cut-off for abnormal the value (defined as 250 ng/mL) than the one we used.
Our results suggest that current diagnostic algorithms should be up-graded to include cardiovascular risk screening and stratification of HIV patients undergoing ART. Given the CVD profile in Africa, characterized by low incidence of ischaemic heart disease and high occurrence of hypertensive heart disease, rheumatic heart disease and cardiomyopathies (40), tailored management protocols need to be developed, including referral pathways adapted to local infrastructures and health professionals availability.
After 36 months five out of 70 patients died. Higher early mortality in HIV patients starting ART in sSA, compared to those in Europe and North America, is well documented (7,41). This might be related to infectious comorbidities and lower access to good quality care, but the role of cardiovascular risk factors and the significance of high plasma D-dimers need to be clarified.
Unmeasured environmental risks and poverty may contribute to the pro-coagulant status. In Mozambique, approximately 95% of households burn solid fuels for cooking (42), mostly wood and charcoal. Long- and short-term exposure to air pollution has been related to rise in markers of inflammation and fibrinolysis (43-45), and social disparities determine different exposure to indoor air pollution (45). In a study including 27% of participants of black ethnicity, Hajat et al. (43) examined the progression to subclinical and clinical CVD among adults free from disease at baseline, and found a positive association between air pollutants and D-dimer.
We could not perform D-dimers on follow up of our patients. ART has been shown to reduce both D-dimers levels and thromboembolic events. D-dimers steadily decreased from a median level 624 to 214 after 12 months of ART in a cohort of 119 Italian HIV-positive treatment-naïve patients with less than 200 CD4 cells/mL at baseline (46). D-dimers blood levels improvement occurs as soon as 6 months after ART initiation (47). Whether lowering the elevated levels of D-dimer in susceptible population groups by lifestyle or pharmacologic means will lower cardiovascular morbidity and mortality still needs confirmation by randomized controlled trials.
Innovative care delivery models including use of point-of-care diagnostics—such as portable ultrasound and rapid tests for biomarkers we used in this study—may support comprehensive NCD care, by allowing prompt diagnosis and management of comorbidities at peripheral health facilities in resource-limited settings. On the other hand, task-shifting of some specialist competences in cardiovascular care to other health professionals may allow decentralization of care and upgrading of HIV surveillance to include selected NCDs, thus creating integrated NCD-HIV prevention, care, treatment and surveillance models.
While we acknowledge that the results of this descriptive study on patients recruited consecutively in an urban health facility are not generalizable, they raise concern and should call our attention to the urgent need to monitor the occurrence of comorbidities and the trends in risk factors for NCD in HIV-infected individuals. Such efforts are needed to sustain the gains made to reduce the number of lives lost to HIV and to improve quality of life of persons living with HIV, thus contributing to meeting United Nations Sustainable Development Goals in Africa.
ConclusionsOther Section
High cardiovascular risk was present in HIV-infected ART-naïve patients from a low income setting in sSA, highlighting the need for active CVD screening and training of health professionals for case management at ART initiation in this continent. Research into conventional and geographically relevant risk factors and burden of CVD is warranted to allow tailoring of cardiovascular risk stratification in HIV-infected individuals from this region. This knowledge will be important to effectively plan and organize the chain of services needed to increase patient survival free of major adverse events and to sustain the gains from massive global and local investments on prevention and management of HIV.
AcknowledgmentsOther Section
We acknowledge our colleagues Naisa Manafe and Rosália Matimbe from Hospital de Mavalane and Instituto Nacional de Saúde in Mozambique, for their support during the implementation of the study.
Funding: This publication resulted from research supported by the University of California, San Diego, Center for AIDS Research (CFAR), an NIH-funded program (P30 AI036214), which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK.
FootnoteOther Section
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt.2019.12.02). AOM serves as an unpaid associate and guest editor of Cardiovascular Diagnosis and Therapy from Jul 2019 to Jun 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The national bioethics committee approved the study. Informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- WHO. Guideline on when to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. Geneva: WHO, 2015.
- Nsanzimana S, Kanters S, Mills E. Towards Test and Treat Strategy for HIV in Sub-Saharan Africa. BMJ 2015;351:h6839. [Crossref] [PubMed]
- UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. Geneva: 2014.
- Etard JF, Ndiaye I, Thierry-Mieg M, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS 2006;20:1181-9. [Crossref] [PubMed]
- Miiro G, Todd J, Mpendo J, et al. Reduced morbidity and mortality in the first year after initiating highly active anti-retroviral therapy (HAART) among Ugandan adults. Trop Med Int Health 2009;14:556-63. [Crossref] [PubMed]
- Munderi P, Watera C, Nakitingi J, et al. Survival and causes of death, 2 years after the introduction of antiretroviral therapy in Africa: a historical cohort comparison in Entebbe, Uganda. Sixteenth International AIDS Conference; Toronto: 2006.
- Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet 2006;367:817-24. [Crossref] [PubMed]
- Uthman OA, Yahaya V, Ashfaq I, et al. A trend analysis and sub-regional distribution in number of people living with HIV and dying with TB in Africa, 1991 to 2006. Int J Health Geogr 2009;8:65. [Crossref] [PubMed]
- Bussmann H, Wester CW, Ndwapi N, et al. Five-year outcomes of initial patients t9eated in Botswana’s National Antiretroviral treatment program. AIDS 2008;22:2303-11. [Crossref] [PubMed]
- Theuring S, Mugeny K, Rubaihayo J, et al. Antiretroviral therapy programme retention and outcomes after 12 months in a retrospective patient cohort in Fort Portal, Uganda: the ongoing challenge of male ART performance. AIDS Clin Res 2015;6:423.
- Ntsekhe M, Mayosi BM. Cardiac manifestations of HIV infection: an African perspective. Nat Clin Pract Cardiovasc Med 2009;6:120-7. [Crossref] [PubMed]
- Nkambule BB, Davison GM, Ipp H. The evaluation of platelet indices and markers of inflammation, coagulation and disease progression in treatment-naïve, asymptomatic HIV-infected individuals. Int J Lab Hematol 2015;37:450-8. [Crossref] [PubMed]
- Bibas M, Biava G, Antinori A. HIV-Associated Venous Thromboembolism. Mediterr J Hematol Infect Dis 2011;3:e2011030. [Crossref] [PubMed]
- Feldblum PJ, Enosse S, Dubé K, et al. HIV prevalence and incidence in a cohort of women at higher risk for HIV acquisition in Chókwè, southern Mozambique. PLoS One 2014;9:e97547. [Crossref] [PubMed]
- Mocumbi AO, Carrilho C, Aronoff-Spencer E, et al. Innovative strategies for transforming internal medicine residency training in resource-limited settings: the Mozambique experience. Acad Med 2014;89:S78-82. [Crossref] [PubMed]
- Gomes A, Damasceno A, Azevedo A, et al. Body mass index and waist circumference in Mozambique: urban/rural gap during epidemiological transition. Obes Rev 2010;11:627-34. [Crossref] [PubMed]
- Silva-Matos C, Gomes A, Azevedo A, et al. Diabetes in Mozambique: prevalence, management and healthcare challenges. Diabetes Metab 2011;37:237-44. [Crossref] [PubMed]
- Damasceno A, Azevedo A, Silva-Matos C, et al. Hypertension prevalence, awareness, treatment, and control in Mozambique: urban/rural gap during epidemiological transition. Hypertension 2009;54:77-83. [Crossref] [PubMed]
- Jessen N, Damasceno A, Silva-Matos C, et al. Hypertension in Mozambique: trends between 2005 and 2015. J Hypertens 2018;36:779-84. [Crossref] [PubMed]
- Mozambique STEPS Survey 2005. Available online: accessed 28th January 2019www.who.int/ncds/surveillance/steps/2005MozambiqueFactSheetEN.pdf
- Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2nd edition. Geneva: World Health Organization; 2016. ANNEX 10, WHO clinical staging of HIV disease in adults, adolescents and children. Available online: https://www.ncbi.nlm.nih.gov/books/NBK374293/
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014;311:1117-24. [Crossref] [PubMed]
- Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med 2009;17:118-23. [PubMed]
- Carr A. HIV lipodystrophy: risk factors, pathogenesis, diagnosis and management. AIDS 2003;17 Suppl 1:S141-8. [Crossref] [PubMed]
- Anastos K, Lu D, Shi Q, et al. Association of serum lipid levels with HIV serostatus, specific antiretroviral agents, and treatment regimens. J Acquir Immune Defic Syndr 2007;45:34-42. [Crossref] [PubMed]
- Lichtenstein KA, Armon C, Buchacz K, et al. HIV Outpatient Study (HOPS) Investigators. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis 2010;51:435-47. [Crossref] [PubMed]
- Savès M, Chêne G, Ducimetière P, et al. French WHO MONICA Project the APROCO (ANRS EP11) Study Group. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis 2003;37:292-8. [Crossref] [PubMed]
- DAD Study Group, Friis-Møller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007;356:1723-35. [Crossref] [PubMed]
- Holmberg SD, Moorman A, Williamson J, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet 2002;360:1747-8. [Crossref] [PubMed]
- Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 2005;352:48-62. [Crossref] [PubMed]
- Patel P, Sabin K, Godfrey-Faussettet P. Approaches to Improve the Surveillance, Monitoring, and Management of Noncommunicable Diseases in HIV-Infected Persons: Viewpoint. JMIR Public Health Surveill 2018;4:e10989. [Crossref] [PubMed]
- Lau B, Gange SJ, Moore RD. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr 2007;44:179-87. [Crossref] [PubMed]
- Patel P, Rose CE, Collins PY, et al. Non-communicable diseases among HIV-infected persons in low-income and middle-income countries: a systematic review and meta-analysis. AIDS 2018;32 Suppl 1:S5-20. [Crossref] [PubMed]
- Munthali C, Taegtmeyer M, Garner PG, et al. Diagnostic accuracy of the WHO clinical staging system for defining eligibility for ART in sub-Saharan Africa: a systematic review and meta-analysis. J Int AIDS Soc 2014;17:18932. [Crossref] [PubMed]
- Kiser KL, Badowski ME. Risk factors for venous thromboembolism in patients with human immunodeficiency virus infection. Pharmacotherapy 2010;30:1292-302. [Crossref] [PubMed]
- Baker JV, Neuhaus J, Duprez D, et al. INSIGHT SMART Study Group Changes in inflammatory and coagulation biomarkers: A randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr 2011;56:36-43. [Crossref] [PubMed]
- Kearon C, Ginsberg JS, Douketis J, et al. Management of suspected deep venous thrombosis in outpatients by using clinical assessment and D-dimer testing. Ann Intern Med 2001;135:108-11. [Crossref] [PubMed]
- Khaleghi M, Saleem U, McBane RD, et al. African American Ethnicity is Associated with Higher Plasma Levels of D-dimer in Adults with Hypertension. J Thromb Haemost 2009;7:34-40. [Crossref] [PubMed]
- Di Stefano M, D’Andrea G, Zoboli F, et al. Preliminary Data From the Study of Coagulative Profile of HIV Infected Individuals Suggest a Role For Point Mutations in the Gene in Protein S Deficiency in Individuals Undergoing Highly Antiretroviral Therapy. Open AIDS J 2018;12:6-10. [Crossref] [PubMed]
- Damasceno A, Mayosi BM, Sani M, et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med 2012;172:1386-94. [Crossref] [PubMed]
- Boulle A, Schomaker M, May MT, et al. Mortality in Patients with HIV-1 Infection Starting Antiretroviral Therapy in South Africa, Europe, or North America: A Collaborative Analysis of Prospective Studies. PLoS Med 2014;11:e1001718. [Crossref] [PubMed]
- Bonjour S, Adair-Rohani H, Wolf J, et al. Solid fuel use for household cooking: country and regional estimates for 1980-2010 Environ. Health Perspect 2013;121:784-90. [Crossref] [PubMed]
- Hajat A, Allison M, Diez-Roux AV, et al. Long-term Exposure to Air Pollution and Markers of Inflammation, Coagulation, and Endothelial Activation: A Repeat-measures Analysis in the Multi-Ethnic Study of Atherosclerosis (MESA). Epidemiology 2015;26:310-20. [Crossref] [PubMed]
- Delfino RJ, Staimer N, Tjoa T, et al. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environ Health Perspect 2009;117:1232-8. [Crossref] [PubMed]
- Hajat A, Hsia C, O'Neill MS. Socioeconomic Disparities and Air Pollution Exposure: a Global Review. Curr Environ Health Rep 2015;2:440-50. [Crossref] [PubMed]
- Maggi P, Bellacosa C, Leone A, et al. Cardiovascular risk in advanced naïve HIV-infected patients starting antiretroviral therapy: Comparison of three different regimens - PREVALEAT II cohort. Atherosclerosis 2017;263:398-404. [Crossref] [PubMed]
- Arildsen H, Sørensen KE, Ingerslev JM, et al. Endothelial dysfunction, increased inflammation, and activated coagulation in HIV-infected patients improve after initiation of highly active antiretroviral therapy. HIV Med 2013;14:1-9. [Crossref] [PubMed]


