Detailed cross-sectional study of 60 superficial femoral artery occlusions: morphological quantitative analysis can lead to a new classification
Introduction
Peripheral arterial occlusive disease (PAOD) is clinically categorized using Fontaine or Rutherford classifications. The first widely used standardized morphological classification was the TransAtlantic Inter-Society Consensus (TASC) for the management of PAOD (TASC I) in 2000 (1). The objective was to create a uniform system to describe arterial lesions based on the morphological evaluation of 2D angiograms, in order to harmonize therapeutic management and guide decision making in patients presenting identical conditions.
TASC I classification was promptly found insufficient and has been updated in 2007 (2) to get on par with the technical advances in endovascular surgery. In this updated classification, femoro-popliteal lesions are divided in four groups (Table 1). TASC II is the only widely accepted taxonomy, and has demonstrated prognostic value for revascularization outcome in superficial femoral artery (SFA) occlusion (3-5). Even though this classification permitted to create a rough guidance for femoro-popliteal lesions depending on their morphology, it has shown some limitations. Indeed, complex arterial lesions are difficult to classify, leading to a poor inter and intra observer reproducibility (6), as only one lesion (stenosis or occlusion) is taken into account for the staging, whereas most peripheral arterial diseases are characterized by a combination of various lesions (7). Moreover, regarding the specific subject of SFA occlusion, TASC II classification is strictly limited to length and calcification analysis.
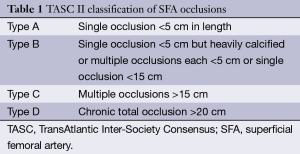
Full table
Non-invasive diagnosis of PAOD can be made by duplex ultrasonography, magnetic resonance angiography (MRA) and computed tomography angiography (CTA) (8). These techniques have gained wide acceptance upon the last ten years, and are mature enough to fully replace conventional diagnostic digital subtraction angiography (DSA) (9-12). Therefore, decision making is nowadays rarely made on DSA and rely more and more on modern cross-sectional imaging (13).
Offering a high image quality, a wide availability even in the emergency setting, a low complication rate and a reasonable cost (10,11), CTA has become the first-line examination for PAOD in many institutions, completely replacing diagnostic DSA. In the particular case of SFA occlusion, CTA can precisely depict its extent, accurately characterize its constitution and evaluate the quality of collateral circulation (14). State-of-the-art CTA can thus provide much more information than traditional invasive angiography (15), such as artery diameter, occlusion length, precise location (central or peripheral) of calcifications and their accurate quantification (16).
We hypothesize that these morphological characteristics given by extensive post-treatment of CTA images could be the basis of a refined classification of SFA occlusions.
Methods
Our institutional review board approved this retrospective study and waived the requirement for informed consent.
Population
Two hundred and thirty two lower limb CTA examinations acquired from January to April, 2013 were reviewed in search of a total or segmental SFA occlusion. Fifty-one CTA examinations with at least one SFA occlusion were found, among which five were excluded for technical problems (hip prosthesis with major metal artifacts in three cases, poor opacification in one case and loss of submillimetric axial slices on PACS in one case). Forty-six CTA examinations corresponding to 46 distinct patients were thus finally included, totalizing 60 SFA occlusions. For each patient, clinical presentation at the time of the examination using the Fontaine classification was documented.
CTA acquisition protocol
All examinations were performed on the same computed tomography (CT) scanner (Discovery CT750HD, GE Healthcare, Milwaukee, Wisconsin, USA). Patient was positioned supine, feet first, and an initial scout view was made for exact planning of volume acquisition, from diaphragm to toes (10). A total of 100 to 125 mL of a high concentration iodine contrast media [iomeprol 400 mg/mL (Iomeron, Bracco, Milan, Italy)] were injected at a high rate (3.5 to 5 mL/sec) using an automatic power injector with a triphasic protocol and a saline chaser. Goal was to adapt the contrast media injection to the scan time, i.e., 25 to 35 seconds depending on patient’s height. Volume acquisition was triggered with a bolus-tracking technique, using a region of interest placed within the abdominal aorta and a starting threshold at 150 HU. CT parameters were set at 100 kVp with automatic tube current modulation, a 0.5 s rotation time, a 0.7 pitch and a 64 mm × 0.625 mm beam collimation. Axial contiguous 0.625 mm thick slices were reconstructed with a high definition filter and used for SFA analysis.
Radiation dose was evaluated with the dose-length product, expressed in mGy.cm.
Image post-processing
All post-processing were carried out on a dedicated Advantage Windows workstation five (GE Healthcare, Milwaukee, Wisconsin, USA) using AdW software in version 11.6.
DSA-like images of the arterial vasculature obtained after bone-removal and rendered using Maximum Intensity Projection and 3D Volume Rendering (17) were read by one experienced radiologist to categorize the SFA occlusions according to TASC II classification.
A curved multiplanar reconstruction (MPR) following the occluded SFA course was drawn by placing center points every five slices (Figure 1) and used to precisely quantify:
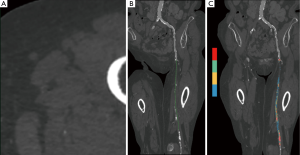
- Total occlusion length from the beginning of the occlusion up to the recovery. In case of popliteal involvement, occluded segment of the popliteal artery in direct continuation of the occluded SFA was included in the measurement;
- Mean diameter of the occluded segment, obtained by the averaging of at least four manual measurements on transverse cross-sections perpendicular to the centerline; mean diameter of the closest upstream permeable arterial segment was used as a comparison;
- Exact amount of calcifications in the occluded segment, using color-coded maps with automatic segmentation based on HU density: voxels in the range 400-3,000 HU were considered as calcifications and their total volume was expressed in cubic millimeter. To obtain a relative quantification of calcifications, the volume of the occluded segment was equated to the one of a cylinder whose length is the total occlusion length and whose diameter is the mean occluded segment diameter. Absolute volume of calcifications, automatically given by the post processing software using the color-coded map, was divided by this occluded volume so as to obtain an average percentage of calcifications within the occluded segment.
These quantitative measures were done by the same experienced radiologist. Mean post-processing time was 15 minutes per artery.
Curved MPR as well as axial slices were used for visual qualitative assessment of (Figure 2):
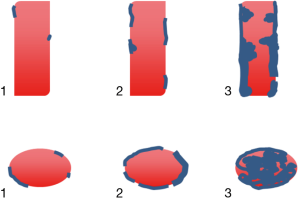
- The global amount of calcifications, using a 3-level qualitative scale: 1, absent to low; 2, moderate; 3, important;
- The characterization of the calcifications based on their axial location: 1, parietal non concentric; 2, parietal concentric; 3, central. Only the higher grade was taken into account in case of multiple types of calcifications.
Statistical analysis
Potential correlations between clinical staging and CT quantitative and qualitative parameters (occlusion length, mean occluded SFA diameter, total volume of calcifications and type of calcifications) were sought using the software MedCalc in version 12.4.0. Pearson test, or Spearman test in case of non-parametric data, were used.
Results
Population
The 46 patients included (30 men, 16 women) were aged 68.3±11.7 years old and their clinical PAOD classification was stage II in 49% of cases and stage III/IV in 51%. Regarding their 60 SFA occlusions, the vast majority (75%) was TASC II stage D (Table 2).
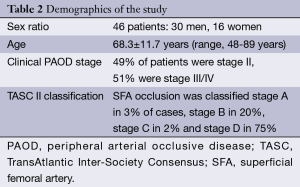
Full table
Radiation dose
Mean Dose-Length Product was 1,395±462 mGy.cm.
CTA quantitative parameters
Mean occlusion length was 219±107 mm, with 39% of all SFA occlusions being total. Mean occluded diameter was 6.1±1.6 mm and the average percentage of calcifications was calculated at 17.4%±20% (Table 3).
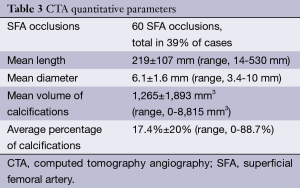
Full table
CTA qualitative parameters
Calcifications were considered absent to low in 22% of cases, moderate in 19% and major in 59%. Regarding their preponderant axial location, they were parietal and non-concentric in 31% of cases, parietal and concentric in 15% and central in 54%.
The relationship between the qualitative visual assessment of the amount of calcifications and the percentage of calcifications is given for various thresholds in Table 4.
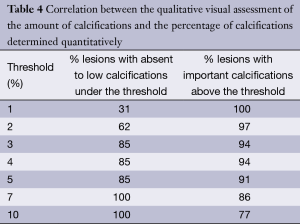
Full table
Correlation
Correlation coefficients between CTA characteristics, clinical staging, age and sex are given in Table 5. There was no significant correlation, with all P being largely above 0.05.

Full table
Discussion
To the best of our knowledge, this study is the first one to use advanced cross-sectional imaging to characterize and classify SFA occlusions.
The goal was to take advantage of all the information provided by modern cross-sectional imaging to bring out relevant anatomical characteristics and demonstrate the variety of SFA occlusions, variety that may appear obvious but is poorly recognized in the literature. TASC II classification is indeed strictly limited to length and calcification analysis, with the distinction between low and “heavily calcified” occlusions only on 2D angiograms a potential source of difficulty. Moreover, assessing the chronicity of an occlusion on a DSA, as stated in TASC II guidelines, is at least challenging and can be a source of inter-observer disagreement.
We chose to use CTA over MRA in this study mainly because in our local guidelines, CTA is the default first-line examination in the acute setting as well as in outpatients. Indeed, CTA is more easily and readily available, due to a severe lack of magnetic resonance (MR) scanner in France, and more cost-effective.
CTA offers major advantages over 2D DSA: it can measure the average diameter of the occlusion, accurately establish the amount of calcifications and determine their exact position relatively to the lumen. Additionally, the quantitative parameters described in this study are independent from the reader, and could offer strong inter- and intra-reader reproducibility, thus addressing one of the most important limitations of TASC II classification.
Based on our observations on these 60 SFA occlusions, we decided to focus on two simple quantitative parameters to categorize these lesions: the mean diameter of the occluded segment and the percentage of calcifications.
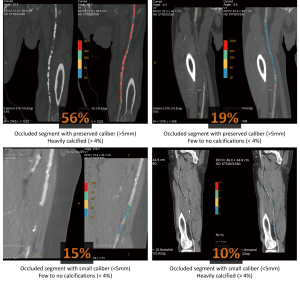
Regarding the mean diameter, they were roughly two types of occlusions: the one where the occluded vessel was shrinked and the one where the diameter was kept normal or slightly enlarged. In accordance with one anatomical study (18), we selected a 5 mm threshold to differentiate SFA occlusions with a preserved diameter (≥5 mm, 73% of cases) from those with a reduced diameter (<5 mm, 27%). Comparison with the nearest upstream permeable arterial segment showed that the occluded vessel is of smaller diameter in 80% of cases when under the 5 mm threshold, whereas only 45% of occluded segments of more than 5 mm are smaller than the adjacent upstream permeable artery. A reduced diameter could be the result of an evolution from a thrombotic to a fibrosclerostic material, thus complicating recanalization procedures. As true axial diameters cannot be determined with 2D angiograms, this parameter is not mentioned in TASC II classification and poorly investigated in the literature.
To determine the level of percentage of calcifications that could best reflect the difference between low and heavily calcified occlusions, we looked at the correlation between the qualitative visual assessment of the amount of calcifications made by two radiologists and the percentage of calcifications determined quantitatively using extensive post processing (Table 4). The objective was to determine an optimal threshold of this percentage, in which calcifications qualitatively rated as absent to low would be under it, and calcifications visually categorized as important above. As demonstrated in Table 4, a 4% threshold offers the best cut-off between low and heavily calcified occlusions, successfully classifying respectively 85% and 94% of them. Moreover, this threshold tends to be associated with a central pattern of calcifications, as 59% of occluded SFA above this cut-off had this type of calcifications. It has been shown that moderate to severe calcifications at the site of re-entry is a predictor of failure in subintimal recanalization (19), but this was assessed using DSA. A central pattern is an original characteristic only evaluable with CTA, and not yet reported in the literature. Florid calcifications can take a staghorn appearance, and if it has still to be proven, it should logically complicate intraluminal or subintimal percutaneous recanalization.
Even in heavily calcified vessels, calcium blooming artifact was not a problem, as the minimal mean diameter of the occluded segment (3.4 mm) was well over the submillimetric spatial resolution of the examination. Therefore, qualitative visual analysis as well as segmentation of the calcifications was optimal.
Combining these two simple quantitative parameters, we could define four groups of patients, as exposed in Table 6 and illustrated in Figure 3.
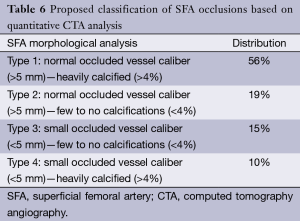
Full table
The absence of correlation between any CTA parameters (diameter of the occluded segment, percentage of calcifications, central or concentric nature of calcifications) and the clinical severity of the disease as expressed by the Fontaine classification is somewhat logical, as the clinical consequence of an SFA occlusion is not related to its nature but to the quality of the collateral circulation. This could explain why our proposed classification is independent from the clinical staging. We also tried to see if the relative proportions of these four types remain the same within the different TASC II groups, but we were limited by the lack of patients in stage A and C. In stage B lesions (n=12), type 1 occlusions remain the most frequent pattern with 42%, whereas type 3 and 4 were the least frequent at 17% each. In stage D lesions (n=45), the distribution was roughly the same as the whole population, with type 1 lesions being the most frequent ones at 59%, and type 4 the rarest at 7%.
This could be particularly useful in the management of TASC II type D lesions: new endovascular revascularization techniques are arising for these common lesions (20,21), and this CTA-based morphological classification could provide support in choosing between them.
Could this study be applied to MRA? State-of-the art MRA can also accurately characterize stenosis and occlusion and provide a precise road-map for treatment planning, with the advantage of a radiation-free examination and immediate DSA-like images without the need of a time consuming bone removal process (22). In addition, the high contrast resolution of Magnetic Resonance Imaging (MRI) could help in distinguishing the different layers of the vessel wall (23,24), possibly offering another way of describing SFA occlusions. Furthermore, MRI can access a more functional aspect of the PAOD, such as the evaluation of the muscle response to a physical stress. Dedicated MR techniques like first-pass perfusion imaging (24-26) or 31PMR spectroscopy (24,27) could offer a quantitative evaluation of the quality of collateral circulation, indirectly reflecting the tolerance and the adaptation to the SFA occlusion. These parameters could prove useful for decision making.
However, in this specific objective of studying SFA occlusions, MRA have one major disadvantage over CTA: its inability to precisely quantify calcifications. Moreover, MRA slices are usually thicker than CTA (2 to 3 mm versus 0.625 mm) resulting in curved MPR of lesser quality (Figure 4), and the cost of MRA is significantly higher (28). Taking all these points into account, we believe that the isotropic volume acquisition and the high calcium contrast obtained with CTA makes it, at least for now, more appropriate.
Study limitations
Being a purely descriptive anatomical study which was not intended to establish any prognosis parameters, its retrospective design and the absence of correlation with therapeutic outcome limit its use in practice. However, the aim of this work was only anatomical and descriptive, and a prospective study is currently under way to test a potential predictive value of these quantitative and qualitative CTA parameters for revascularization success.
Post-processing of each occluded artery was done by only one experiences radiologist: it would have been interesting to have the CT data post-treated by two different radiologists to study the inter-observer agreement. Our preliminary tests in five different patients show however that inter-observer agreement is excellent.
The calculation of the percentage of calcifications remains an approximation, as the occluded vessel is not in reality a perfect cylinder. This original parameter is however simple to obtain and, even though not perfectly accurate, gives a good approximation of the global amount of calcifications in the occluded artery.
CTA is now a mature and robust imaging examination that has totally overcome diagnostic DSA and can be considered as the reference exploration of lower limb arteries. Provided adequate acquisition and expert post-treatment are carried out, CTA can bring numerous advantages in depiction and characterization of SFA diseases. Thus, simple morphological elements such as occluded vessel diameter and percentage of calcifications can lead to a new approach in the classification of SFA occlusions. Not all occlusions are the same, and this statement, as obvious as it may seem, is elegantly proven and exposed by cross-sectional imaging, and could be the basis of a more precise patient management.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg 2000;31:S1-S296. [PubMed]
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45 Suppl S:S5-67.
- Al-Khoury G, Marone L, Chaer R, et al. Isolated femoral endarterectomy: impact of SFA TASC classification on recurrence of symptoms and need for additional intervention. J Vasc Surg 2009;50:784-9. [PubMed]
- Ihnat DM, Duong ST, Taylor ZC, et al. Contemporary outcomes after superficial femoral artery angioplasty and stenting: the influence of TASC classification and runoff score. J Vasc Surg 2008;47:967-74. [PubMed]
- Faglia E, Clerici G, Airoldi F, et al. Revascularization by angioplasty of type D femoropopliteal and long infrapopliteal lesion in diabetic patients with critical limb ischemia: are TASC II recommendations suitable? A population-based cohort study. Int J Low Extrem Wounds 2012;11:277-85. [PubMed]
- Kukkonen T, Korhonen M, Halmesmäki K, et al. Poor inter-observer agreement on the TASC II classification of femoropopliteal lesions. Eur J Vasc Endovasc Surg 2010;39:220-4. [PubMed]
- Ricco JB. Advantages and limitations of TASC II classification of femoropopliteal lesions. Eur J Vasc Endovasc Surg 2010;39:225-6. [PubMed]
- Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ 2007;334:1257. [PubMed]
- Clair D, Shah S, Weber J. Current state of diagnosis and management of critical limb ischemia. Curr Cardiol Rep 2012;14:160-70. [PubMed]
- Kayhan A, Palabıyık F, Serinsöz S, et al. Multidetector CT angiography versus arterial duplex USG in diagnosis of mild lower extremity peripheral arterial disease: is multidetector CT a valuable screening tool? Eur J Radiol 2012;81:542-6. [PubMed]
- Osama A, Zaytoun H, Soliman HH. Role of multi-slice CT angiography versus Doppler ultrasonography and conventional angiography in assessment of aorto-iliac arterial disease. The Egyptian Journal of Radiology and Nuclear Medicine 2012;43:561:73.
- Sommer WH, Bamberg F, Johnson TR, et al. Diagnostic accuracy of dynamic computed tomographic angiographic of the lower leg in patients with critical limb ischemia. Invest Radiol 2012;47:325-31. [PubMed]
- Fotiadis N, Kyriakides C, Bent C, et al. 64-section CT angiography in patients with critical limb ischaemia and severe claudication: comparison with digital subtractive angiography. Clin Radiol 2011;66:945-52. [PubMed]
- Heijenbrok-Kal MH, Kock MC, Hunink MG. Lower extremity arterial disease: multidetector CT angiography meta-analysis. Radiology 2007;245:433-9. [PubMed]
- Kashyap VS, Pavkov ML, Bishop PD, et al. Angiography underestimates peripheral atherosclerosis: lumenography revisited. J Endovasc Ther 2008;15:117-25. [PubMed]
- Lell MM, Anders K, Uder M, et al. New Techniques in CT Angiography. RadioGraphics 2006;26:S45-S62. [PubMed]
- Fishman EK, Ney DR, Heath DG, et al. Volume rendering versus maximum intensity projection in CT angiography: what works best, when, and why. Radiographics 2006;26:905-22. [PubMed]
- Kröger K, Buss C, Goyen M, et al. Diameter of occluded superficial femoral arteries limits percutaneous recanalization: preliminary results. J Endovasc Ther 2002;9:369-74. [PubMed]
- Shin SH, Baril D, Chaer R, et al. Limitations of the Outback LTD re-entry device in femoropopliteal chronic total occlusions. J Vasc Surg 2011;53:1260-4. [PubMed]
- Ramaiah V. Endovascular infrainguinal revascularization: technical tips for atherectomy device selection and procedural success. Semin Vasc Surg 2008;21:41-9. [PubMed]
- Sharafuddin MJ, Amin PB, Nicholson RM, et al. Femoropopliteal Endovascular Interventions. In: Hoballah JJ, Lumsden AB. eds. Vascular Surgery: Springer London, 2012:213-40.
- Ersoy H, Rybicki FJ. MR Angiography of the Lower Extremities. American Journal of Roentgenology 2008;190:1675-84. [PubMed]
- Mitsouras D, Owens CD, Conte MS, et al. In vivo differentiation of two vessel wall layers in lower extremity peripheral vein bypass grafts: application of high-resolution inner-volume black blood 3D FSE. Magn Reson Med 2009;62:607-15. [PubMed]
- Kramer CM. Peripheral arterial disease assessment: wall, perfusion, and spectroscopy. Top Magn Reson Imaging 2007;18:357-69. [PubMed]
- Richardson RS, Haseler LJ, Nygren AT, et al. Local perfusion and metabolic demand during exercise: a noninvasive MRI method of assessment. J Appl Physiol (1985) 2001;91:1845-53. [PubMed]
- Isbell DC, Epstein FH, Zhong X, et al. Calf muscle perfusion at peak exercise in peripheral arterial disease: measurement by first-pass contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging 2007;25:1013-20. [PubMed]
- Zatina MA, Berkowitz HD, Gross GM, et al. 31P nuclear magnetic resonance spectroscopy: noninvasive biochemical analysis of the ischemic extremity. J Vasc Surg 1986;3:411-20. [PubMed]
- Ouwendijk R, de Vries M, Pattynama PM, et al. Imaging peripheral arterial disease: a randomized controlled trial comparing contrast-enhanced MR angiography and multi-detector row CT angiography. Radiology 2005;236:1094-103. [PubMed]


