Assessing the end-organ in peripheral arterial occlusive disease—from contrast—enhanced ultrasound to blood-oxygen-level-dependent MR imaging
Introduction
Peripheral arterial occlusive disease (PAOD) represents one of the manifestations of generalized atherosclerotic disease and is characterized by mostly slowly progressing stenoses and occlusions of extremity arteries. Prevalence based on ankle brachial index (ABI) measurements is high, being 3% in 45-50 years old men, and up to 18% in 70-75 years old men (1). While the presence of PAOD rarely ends with a critical limb ischemia and loss of tissue as an impeding consequence, it indicates a 20-60% increased risk for myocardial infarction; a 2- to 6-fold increased risk of death due to coronary events and a 4 to 5 times higher risk of a cerebrovascular events (2).
The gold standard for the diagnosis of PAOD is angiography which is hampered by its invasiveness, nephrotoxic contrast agent load and costs. Therefore blood pressure measurements at the ankle (ABI) after history and physical examination is the mainstay of diagnosis nowadays, an ABI value <0.9 indicating the presence of PAOD with a sensitivity and specificity of >90%. The vast majority of PAOD patients are either asymptomatic or may complain of intermittent claudication. While in chronical critical ischemia the skin followed by muscle, bone and nerves is mainly affected, the system that suffers primarily from exercise related reduced perfusion in claudicants is the skeletal muscle. These are the functional end organs of interest in PAOD patients causing the actual clinical symptoms. It is known that in these patients an adaptation of the calf muscle occurs. A change from type I (fast twitch, glycogenolytic) to type II (slow twitch, oxydative) and a reduced number of capillaries have been detected in patients with claudication compared to healthy volunteers (3). On a cellular level an increased rate of apoptosis in the calf muscles of these patients was appreciated (4). These informations could only be gained by biopsy and histophatology analysis. Efforts have been undertaken to understand metabolic and perfusion alterations in the skeletal muscle non-invasively but it remains an open field for research. In the last years new methods have been propagated to study the actual end organ in PAOD, namely the skeletal muscle, using microperfusion imaging techniques, such as contrast enhanced ultrasound (CEUS) and blood-oxygen-level-dependent (BOLD) MR imaging (MRI). In the following article these two methods and the most important new insights gained herewith are described in the clinical setting of PAOD.
Technique of CEUS
The contrast agent used to depict macrocirculation but especially microcirculation and tissue perfusion contains stabilized microbubbles that are infused intravenously. These bubbles consist of a core with sulfur hexafluoride gas surrounded by a phospholipid shell. Unlike contrast agents for cross sectional imaging, CEUS microbubbles do not leave the vascular system. Apart from severe pulmonary hypertension and known allergy against microbubbles there are no absolute contraindications. These microbubbles are not nephrotoxic and can therefore given in patients with renal insufficiency as they are eliminated through the respiratory tract. The ultrasound equipment for vascular examinations regarding probes ranges from low frequency (e.g., 1-5 MHz) to higher frequencies (e.g., 3-9/10 MHz) depending on the necessity of depth of penetration. In order not to destroy the microbubbles a low mechanical index setting CEUS imaging is mandatory (e.g., pulse inversion harmonic imaging). The contrast agent is generally given as a bolus of 2.4 mL but can be reduced to 1 mL or increased to a maximum of 4.8 mL depending on the equipment and the investigated region (5). Especially for tissue perfusion quantification the kinetics of the time intensity curve after bolus injection is analyzed and larger amounts of contrast agent can be necessary. Parameters derived from the intensity curves often used are time-to-peak (TTP), peak intensity, or wash in- and wash out-time. When working with a continuos infusion the disruption-replenish analysis based on an augmentation of the mechanical index for a few frames can be applied to enhance the microperfusion analysis.
CEUS in PAOD
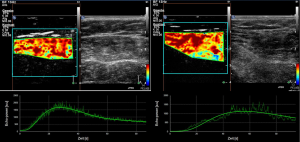
Several groups have applied this technology to study the skeletal muscle under different physiologic and pathological conditions, i.e., different populations (healthy volunteers, patients with PAOD or diabetes mellitus) and with different provocation manoeuvers. The vast majority of examinations are performed at the muscles of the calf as they are relatively easy to depict and are representative for distal limb skeletal muscle tissue. Skeletal muscle microperfusion on CEUS in a healthy volunteer and in patient with PAOD including time-intensity-curves is shown in Figure 1.
Obvious differences in the wash-in-curves at rest were described between healthy people and patients with intermittent claudication (6). The TTP was significantly longer in the patient group. In another study the authors were able to show that TTP also significantly differed within the PAOD patients with shorter TTP in those having good collateral vessel development while ankle/brachial-index did not reveal this difference (7). In diabetic patients the transit time (arrival) from a small artery to a muscle vein in the calf was significantly longer than in PAOD patients or in healthy volunteers (8). Most authors have conducted CEUS with a certain provocation maneuver (similar to the paradigms applied in cross sectional imaging to provoke signals in muscle BOLD MRI or fMRI of the brain) in order to simulate or exacerbate the situation of reduced perfusion in patients with claudication. A further CEUS imaging investigation in long-standing diabetics (more than ten years) applying a cuff compression/decompression paradigma found decreased (though partially not statistical significant) values in maximum signal intensity, slope to maximum and area under the curve in the hyperermia phase in comparison to healthy volunteers and diabetics with a short course (less than ten years) (9). In PAOD patients on the other hand differences after provocation compared to healthy volunteers were overt. TTP was shown to be shorter, slope to the maximum intensity stepper and vascular response after occlusion was more pronounced, all parameters statistically significant different in PAOD patients vs. healthy volunteers (10). Beyond the differentiation between diseased and healthy microvascular state CEUS-parameters as calculated exercise blood flow and flow reserve during or after plantar-flexion exercise even allowed for predicting severity of disease in terms of claudication threshold (11). Finally, successful interventions (bypasses or dilatations) in PAOD patients could be detected based on quantitative CEUS. TTP obtained without provocation maneuvers compared before and after intervention was equivalent to improvement in ankle/brachial-index or pulse volume recordings and may therefore serve as an alternative in certain patients (12).
In summary CEUS of the skeletal muscle is able to differentiate between healthy people and patients with PAOD and gives a unique insight in the microperfusion of the end organ responsible for vascular claudication.
BOLD MRI of skeletal muscle to examine PAOD
The BOLD signal in muscle tissue
In recent years, BOLD MRI has made its way from an extraordinary valuable neuroimaging tool to the functional assessment of skeletal muscle tissue. BOLD imaging relies on the principle that deoxygenated hemoglobin is paramagnetic and thus leads to magnetic field distortions in its vicinity. As a consequence, via an increase of the oxy- to deoxyhemoglobin ratio, for example through increased muscle perfusion, muscle exercise or oxygen inhalation, a decrease of the transverse relaxation rate (R2, R2*) and thus an increase of T2/T2* in muscle tissue occurs. This BOLD effect is even enhanced in gradient echo sequences (13). As initial studies in the late 1990s revealed, this effect is a “true” BOLD effect originating from changes of the intravascular hemoglobin oxygenation rate and not depending on muscular myoglobin oxygenation (14). This finding has been corroborated a few years later by a study showing via arterial signal nulling that no significant extravascular influence on the muscle BOLD signal existed, at least when applying the cuff compression paradigm (15). These findings were in line with studies showing a good correlation between blood flow as measured via laser Doppler flowmetry or arterial spin labeling with BOLD imaging in healthy and diseased study populations (16-19). Microvascular oxygenation state could also be positively correlated with BOLD signal changes (20-22). However, as the oxygenation level of intravascular hemoglobin is not only dependent from oxyhemoglobin supply (i.e., perfusion) and deoxygenation rate of the respective tissue (i.e., metabolic activity) but also influenced by further factors such as blood volume, hemoglobin concentration and metabolic variables (i.e., pH, lactate, phosphate), one has to consider the origin of the BOLD signal as multifactorial (23-26). Additionally, many studies conducted to reveal the underlying physiology of muscle BOLD effect are based on different imaging paradigms, post processing routines or study collectives and thus difficult to interpret (27).
Irrespective of its exact (patho)physiologic and physical origins, it is widely accepted that the skeletal muscle BOLD signal is strongly dependent on microvascular function of muscle tissue and has consequently been analyzed an indicator of small vessel disease in the clinical setting (28).
Evaluating BOLD responses of skeletal muscle tissue
BOLD MRI of skeletal muscles can be performed on standard whole body MR scanners used in the daily clinical routine. As with regular morphologic MR studies, certain contraindications exist, such as claustrophobia, ferromagnetic implants and certain pacemakers. Due to the different variables influencing BOLD response several precautions should be taken into considerations. It should be ensured that the imaged person has rested at least five minutes prior to the beginning of the examination as physical exercise has been shown to modulate the BOLD signal (29). For interpreting BOLD signal changes it is important to consider that vasoactive drugs can influence T2* (30).
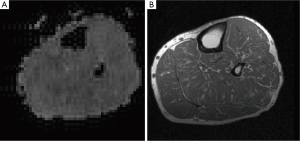
Resting should be performed in a laying position on the examination couch as the vascular filling status of the examined extremities should not be changed shortly prior starting the examination. The extremity is usually placed within a vascular array coil between foam braces which are used to reduce motion artifacts. As BOLD imaging needs high speed acquisition methods, typically echo planar imaging (EPI) methods are applied. EPI maps are often generated from several transverse sections of the respective skeletal muscle to reduce through plane motion artifacts. For each section, regions of interest are placed within the desired skeletal muscles using T1 anatomical reference images (Figure 2). Multi-echo sequences with fat suppression can be used to measure at different echo times and thus reduce inflow artifacts in contrast to conventional single-echo EPI images. Multi-echo-gradient echo sequences are used to separate real oxygenation level dependent T2* signal changes from other influences such as changes of T1, baseline drifts and inflow artifacts (13,27,31).
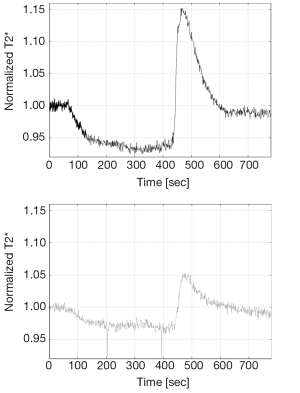
Several different imaging paradigms have been proposed for the functional evaluation of skeletal muscle tissue using BOLD MRI, including arterial occlusion (also known as the ischemia/hyperemia paradigm), muscle exercise and oxygen inhalation. In preclinical studies, arterial occlusion and muscle exercise are the most common used variants. The prevention of blood inflow into the extremity is induced via supra-systolic inflation of an air cuff wrapped around the proximal part of the thigh or upper arm. Especially when studying subjects with vascular diseases such as PAOD applying supra-systolic pressure, it is important to ensure that no active clinical signs of critical limb ischemia are present. During the initial resting period the baseline BOLD signal is determined. After cuff compression, a typical fast ischemic signal decline is observed, followed by a slower decline to the minimum ischemic value or T2* min (Figure 3). Quickly after cuff deflation, hyperemia leads to a T2* increase up to the hyperemia peak value or T2* max after 30-60 seconds. The times to (half) ischemic minimum [T(H)IM] and/or to hyperemic peak (TTP) may be used as further key parameters describing the kinetics of the BOLD time course. Then peaking T2* normally shows a fast signal decline to reach the end value, again reflecting essentially the baseline. Key parameters can be calculated to compare healthy and diseased individuals or to show intra-individual signal changes over time.
Muscle exercise paradigms are making use of the post-contractile blood flow increase in muscle tissue which shows comparable kinetics to the BOLD signal changes observed in activated neuronal tissue (32). Beside voluntary isometric contractions which are most commonly used in this kind of paradigm also electrical stimulation may be used in this setting but represents a challenge due to the necessary metallic materials in the MR environment. Solicited contractions require a high level of compliance from the participant and lead to motion artifacts in the induced time courses. They have however been shown to represent a reliable paradigm in experimental and preclinical skeletal muscle BOLD studies (16,33,34). Those studies showed that even brief contractions lasting only between one and three seconds are sufficient to elicit significant T2* signal changes which are peaking about 10 to 15 seconds after the contraction.
BOLD MRI in PAOD
Arterial stenoses and occlusions in PAOD cause a decrease in blood flow in the extremities leading to a decreased skin and muscle perfusion, which are the functional end-organs. Peripheral arterial macrovascular lesions may be localized and characterized by invasive catheter-directed angiography or magnetic resonance angiography (MRA). However the assessment of the microvasculature at the end-organ level cannot be recorded with purely morphological MRI. BOLD MRI has made an entrance into the literature as a method of muscular oxygenation assessment at the microvascular level (14,35). The technique was studied in different patient populations including PAOD, systemic sclerosis, compartment syndrome. Ledermann et al. compared calf musculature BOLD response during post-ischemic reactive hyperemia in healthy controls and in patients with PAOD, and concluded that there were significant differences in maximal T2* change (∆T2*max, P<0.001) and TTP (P<0.001) between the groups (36). Subsequently, Potthast et al. actually studied the calf musculature BOLD response both in healthy controls and in patients with PAOD during induced ischemia, finding a significantly reduced decrease in signal in the PAOD group during the ischemia phase compared to an age-matched control group (P<0.05) (37). Both of these studies demonstrate the value of muscle BOLD MRI of the skeletal muscle in assessment of microvascular function in patients with PAOD. The disease is not only affecting the macrovasculature, but it also impairs microvasculature on the end-organ level. A representative example of the T2* time course in a healthy volunteer and a PAOD patient is shown in Figure 3. Muscle BOLD MRI might also be of benefit in evaluating post-intervention treatment response in PAOD patients. Huegli et al. conducted a study evaluating calf musculature BOLD signal change prior to and after percutaneous transluminal angioplasty (PTA) of the superficial femoral artery in patients with symptomatic stenoses (38). It was concluded that there was an increase in ∆T2*max (P<0.51) and there were decreases in TTP (P<0.11) and T2* end value (P<0.69) following successful PTA using induced reactive hyperemia. These changes are basically directed towards the normal muscle BOLD response in healthy volunteers, indicating the potential usefulness of the technique in evaluation of treatment approaches at the microcirculatory end organ level. All of these studies have demonstrated promising initial results regarding the use of BOLD MRI in studying the microvascular integrity of the skeletal muscle in PAOD patients.
Reproducibility of results is an important aspect of novel MR techniques when considering applicability in the clinical setting. A study by Versluis et al. addressed the concerns regarding reproducibility by examining patients with PAOD and compared PAOD patients vs. healthy volunteers with calf musculature BOLD MRI (39). Two independent MRI readers analyzed the imaging and the calculated quantitative BOLD data (T2*max and TTP). The reproducibility was found to be poor with a coefficient of variation up to 50.9%. Therefore, additional research needs to be undertaken in order to optimize and standardize this technique for evaluating the skeletal microvasculature in PAOD patients.
Comparison of BOLD and dynamic contrast enhanced MRI (DCE-MRI)
Different techniques exist with regard to skeletal muscle MR microperfusion imaging. In the previous section the BOLD technique was discussed. BOLD MRI is a completely non-invasive technique, even not requiring the administration of contrast agents. DCE-MRI provides information about the skeletal muscle microcirculation by tracking a contrast agent when passing through the muscle tissue and generating based on these data a time-intensity curve. The contrast agent is gadolinium based and injected intravenously. Based on the enhancement pattern reflected in the time-intensity curve information about blood volume, vascular permeability and perfusion can be drawn. This differs from the BOLD technique which is primarily supposed to reflect perfusion induced changes of oxygenation on the muscle tissue level (19,21). Both techniques, BOLD and DCE-MRI have in common that their signal is influenced by a variety of factors. BOLD and DCE-MRI have been applied with promising results in PAOD patients (40). Generally speaking, the ischemia-hyperemia paradigm revealed to play a major role in BOLD MRI whereas different exercise protocols have been established for the use of DCE-MRI (41).
Summary
CEUS imaging and BOLD MRI offer new insights in skeletal muscle microperfusion in PAOD patients. The skeletal muscle is a functional end-organs contributing to the claudication symptoms experienced by PAOD patients. Both, CEUS and BOLD MRI enable quantitative assessment of the skeletal muscle microvasculature. The success of treatment approaches on the end-organ level can be demonstrated using these evolving techniques. The treatment approaches which can be assessed with CEUS or muscle BOLD MRI may also include induction of neovascularization after gene therapies or effects of new vasoactive drugs. Further studies on the microperfusion in larger PAOD patient populations using quantitative CEUS and BOLD imaging are warranted.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kröger K, Stang A, Kondratieva J, et al. Prevalence of peripheral arterial disease - results of the Heinz Nixdorf recall study. Eur J Epidemiol 2006;21:279-85. [PubMed]
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463-654. [PubMed]
- Askew CD, Green S, Walker PJ, et al. Skeletal muscle phenotype is associated with exercise tolerance in patients with peripheral arterial disease. J Vasc Surg 2005;41:802-7. [PubMed]
- Mitchell RG, Duscha BD, Robbins JL, et al. Increased levels of apoptosis in gastrocnemius skeletal muscle in patients with peripheral arterial disease. Vasc Med 2007;12:285-90. [PubMed]
- Staub D, Partovi S, Imfeld S, et al. Novel applications of contrast-enhanced ultrasound imaging in vascular medicine. Vasa 2013;42:17-31. [PubMed]
- Duerschmied D, Olson L, Olschewski M, et al. Contrast ultrasound perfusion imaging of lower extremities in peripheral arterial disease: a novel diagnostic method. Eur Heart J 2006;27:310-5. [PubMed]
- Duerschmied D, Zhou Q, Rink E, et al. Simplified contrast ultrasound accurately reveals muscle perfusion deficits and reflects collateralization in PAD. Atherosclerosis 2009;202:505-12. [PubMed]
- Duerschmied D, Maletzki P, Freund G, et al. Analysis of muscle microcirculation in advanced diabetes mellitus by contrast enhanced ultrasound. Diabetes Res Clin Pract 2008;81:88-92. [PubMed]
- Amarteifio E, Wormsbecher S, Demirel S, et al. Assessment of skeletal muscle microcirculation in type 2 diabetes mellitus using dynamic contrast-enhanced ultrasound: a pilot study. Diab Vasc Dis Res 2013;10:468-70. [PubMed]
- Amarteifio E, Wormsbecher S, Krix M, et al. Dynamic contrast-enhanced ultrasound and transient arterial occlusion for quantification of arterial perfusion reserve in peripheral arterial disease. Eur J Radiol 2012;81:3332-8. [PubMed]
- Lindner JR, Womack L, Barrett EJ, et al. Limb stress-rest perfusion imaging with contrast ultrasound for the assessment of peripheral arterial disease severity. JACC Cardiovasc Imaging 2008;1:343-50. [PubMed]
- Duerschmied D, Maletzki P, Freund G, et al. Success of arterial revascularization determined by contrast ultrasound muscle perfusion imaging. J Vasc Surg 2010;52:1531-6. [PubMed]
- Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990;87:9868-72. [PubMed]
- Lebon V, Carlier PG, Brillault-Salvat C, et al. Simultaneous measurement of perfusion and oxygenation changes using a multiple gradient-echo sequence: application to human muscle study. Magn Reson Imaging 1998;16:721-9. [PubMed]
- Sanchez OA, Copenhaver EA, Elder CP, et al. Absence of a significant extravascular contribution to the skeletal muscle BOLD effect at 3 T. Magn Reson Med 2010;64:527-35. [PubMed]
- Meyer RA, Towse TF, Reid RW, et al. BOLD MRI mapping of transient hyperemia in skeletal muscle after single contractions. NMR Biomed 2004;17:392-8. [PubMed]
- Ledermann HP, Heidecker HG, Schulte AC, et al. Calf muscles imaged at BOLD MR: correlation with TcPO2 and flowmetry measurements during ischemia and reactive hyperemia--initial experience. Radiology 2006;241:477-84. [PubMed]
- Wigmore DM, Damon BM, Pober DM, et al. MRI measures of perfusion-related changes in human skeletal muscle during progressive contractions. J Appl Physiol (1985) 2004;97:2385-94. [PubMed]
- Partovi S, Schulte AC, Staub D, et al. Correlation of skeletal muscle blood oxygenation level-dependent MRI and skin laser doppler flowmetry in patients with systemic sclerosis. J Magn Reson Imaging 2013; [PubMed]
- Ledermann HP, Heidecker HG, Schulte AC, et al. Calf muscles imaged at BOLD MR: correlation with TcPO2 and flowmetry measurements during ischemia and reactive hyperemia--initial experience. Radiology 2006;241:477-84. [PubMed]
- Partovi S, Aschwanden M, Jacobi B, et al. Correlation of muscle BOLD MRI with transcutaneous oxygen pressure for assessing microcirculation in patients with systemic sclerosis. J Magn Reson Imaging 2013;38:845-51. [PubMed]
- Towse TF, Slade JM, Ambrose JA, et al. Quantitative analysis of the postcontractile blood-oxygenation-level-dependent (BOLD) effect in skeletal muscle. J Appl Physiol (1985) 2011;111:27-39. [PubMed]
- Noseworthy MD, Bulte DP, Alfonsi J. BOLD magnetic resonance imaging of skeletal muscle. Semin Musculoskelet Radiol 2003;7:307-15. [PubMed]
- Duteil S, Wary C, Raynaud JS, et al. Influence of vascular filling and perfusion on BOLD contrast during reactive hyperemia in human skeletal muscle. Magn Reson Med 2006;55:450-4. [PubMed]
- Damon BM, Gore JC. Physiological basis of muscle functional MRI: predictions using a computer model. J Appl Physiol (1985) 2005;98:264-73. [PubMed]
- Wilkie DR, Dawson MJ, Edwards RH, et al. 31P NMR studies of resting muscle in normal human subjects. Adv Exp Med Biol 1984;170:333-47. [PubMed]
- Jacobi B, Bongartz G, Partovi S, et al. Skeletal muscle BOLD MRI: from underlying physiological concepts to its usefulness in clinical conditions. J Magn Reson Imaging 2012;35:1253-65. [PubMed]
- Partovi S, Karimi S, Jacobi B, et al. Clinical implications of skeletal muscle blood-oxygenation-level-dependent (BOLD) MRI. MAGMA 2012;25:251-61. [PubMed]
- Towse TF, Slade JM, Meyer RA. Effect of physical activity on MRI-measured blood oxygen level-dependent transients in skeletal muscle after brief contractions. J Appl Physiol (1985) 2005;99:715-22. [PubMed]
- Bulte DP, Alfonsi J, Bells S, et al. Vasomodulation of skeletal muscle BOLD signal. J Magn Reson Imaging 2006;24:886-90. [PubMed]
- Weisskoff RM, Zuo CS, Boxerman JL, et al. Microscopic susceptibility variation and transverse relaxation: theory and experiment. Magn Reson Med 1994;31:601-10. [PubMed]
- Hennig J, Scheffler K, Schreiber A. Time resolved observation of BOLD effect in muscle during isometric exercise. Proc Int Soc Magn Reson Med 2000;8:122.
- Slade JM, Towse TF, Gossain VV, et al. Peripheral microvascular response to muscle contraction is unaltered by early diabetes but decreases with age. J Appl Physiol (1985) 2011;111:1361-71. [PubMed]
- Damon BM, Wadington MC, Hornberger JL, et al. Absolute and relative contributions of BOLD effects to the muscle functional MRI signal intensity time course: effect of exercise intensity. Magn Reson Med 2007;58:335-45. [PubMed]
- Lebon V, Brillault-Salvat C, Bloch G, et al. Evidence of muscle BOLD effect revealed by simultaneous interleaved gradient-echo NMRI and myoglobin NMRS during leg ischemia. Magn Reson Med 1998;40:551-8. [PubMed]
- Ledermann HP, Schulte AC, Heidecker HG, et al. Blood oxygenation level-dependent magnetic resonance imaging of the skeletal muscle in patients with peripheral arterial occlusive disease. Circulation 2006;113:2929-35. [PubMed]
- Potthast S, Schulte A, Kos S, et al. Blood oxygenation level-dependent MRI of the skeletal muscle during ischemia in patients with peripheral arterial occlusive disease. Rofo 2009;181:1157-61. [PubMed]
- Huegli RW, Schulte AC, Aschwanden M, et al. Effects of percutaneous transluminal angioplasty on muscle BOLD-MRI in patients with peripheral arterial occlusive disease: preliminary results. Eur Radiol 2009;19:509-15. [PubMed]
- Versluis B, Backes WH, van Eupen MG, et al. Magnetic resonance imaging in peripheral arterial disease: reproducibility of the assessment of morphological and functional vascular status. Invest Radiol 2011;46:11-24. [PubMed]
- Leppek R, Hoos O, Sattler A, et al. MR-Imaging of lower leg muscle perfusion. Herz 2004;29:32-46. [PubMed]
- Jiji RS, Pollak AW, Epstein FH, et al. Reproducibility of rest and exercise stress contrast-enhanced calf perfusion magnetic resonance imaging in peripheral arterial disease. J Cardiovasc Magn Reson 2013;15:14. [PubMed]


