Management of gastrointestinal bleeding in patients anticoagulated with dabigatran compared with warfarin: a retrospective, comparative case review
Introduction
Dabigatran etexilate is a novel oral anticoagulant, which was approved by the Food and Drug Administration (FDA) for stroke and systemic emboli prevention in patients with non-valvular atrial fibrillation. It pharmacodynamically acts as a direct thrombin inhibitor resulting in a predictable anticoagulation effect, thus, eliminating the need for frequent laboratory monitoring (1,2). Other advantages of dabigatran include fewer drug interactions and short onset of action (2). Moreover, the randomized evaluation of long-term anticoagulation therapy (RE-LY) trial demonstrated the superiority of dabigatran in stroke and systemic emboli prevention over warfarin and also showed the lower risk of overall, life-threatening, and intracranial bleeding in dabigatran group compared with warfarin (3). Yet, the study revealed a higher risk of gastrointestinal (GI) bleeding and dyspepsia in dabigatran group compared with warfarin (3). Additionally, there have been reported cases of massive GI bleeding from dabigatran in elderly patients and patients with moderate renal impairment (4-9). Considering these findings and the fact that accurate laboratory monitoring and antidote are not available, the concerns over the management and outcome of patients with GI bleeding caused by dabigatran has been increased.
Unlike dabigatran, treatment for GI bleeding due to warfarin has been well established. Multiple options for reversing warfarin are available depending on severity of bleeding and level of anticoagulation such as vitamin K for slow reversal and fresh frozen plasma (FFP) or activated factor VII for rapid reversal. Contrarily, patients with GI bleeding from dabigatran are managed conservatively without any proven reversal agents available (10). Due to the differences in pharmacodynamics, pharmacokinetics, and treatment modalities of these two medications, we aim to explore differences in clinical outcomes, number of packed red blood cell (PRBC) transfusions, and length of stay of patients who were hospitalized due to GI bleeding caused by dabigatran compared with warfarin.
Methods
We conducted a retrospective study to compare clinical outcomes and length of stay in hospitalized GI bleeding patients who were on dabigatran compared with those who were on warfarin with therapeutic international normalized ratio (INR) level for non-valvular atrial fibrillation. The study protocol was reviewed and approved by St John Hospital and Medical Center’s institutional review board. We searched for patients hospitalized for GI bleeding who were taking either dabigatran or warfarin for non-valvular atrial fibrillation at St John Hospital and Medical Center during 2009 to 2012 using ICD-9 codes among records in our data warehouse. Exclusion criteria included patients who had coagulation disorders, history of cirrhosis, history of active alcohol use, and platelet count lower than 50,000/microliter. Patients in the warfarin group who had INR level less than two or more than three were also excluded from the study. Demographic data, including age, sex, race, body mass index (BMI), and past medical history were obtained. History of previous GI bleeding, previous GI pathology, and medications that affect coagulation such as aspirin, clopidogrel, non-steroidal anti-inflammatory drugs (NSAIDs) were also documented. History of sick sinus syndrome, beta-blocker, calcium-channel blocker, and other anti-arrhythmic drugs were obtained. Clinical data and laboratory data at presentation and during hospitalization were collected, including presence of tachycardia, presence of hypotension, hemoglobin at presentation, second hemoglobin within 24 hours after presentation, creatinine level at presentation, presence of acute kidney injury, INR level, activated partial thromboplastin time (aPTT) level, thrombin time (TT) level, number of PRBC transfusions, the need for intensive care unit (ICU) admission, length of stay, and death. The data between the two groups were compared.
Creatinine clearance (CrCl) calculated by Cockcroft-Gault equation was utilized for selection of dabigatran dose according to the FDA recommendation and the RE-LY trial (3). Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was utilized to calculate CrCl for the analysis considering CKD-EPI is more accurate in estimating CrCl in elder population and patients with CrCl >60 mL/min (11,12). The staging of chronic kidney disease (CKD) was then classified according to Kidney Disease Outcomes Quality Initiative (KDOQI) guideline (13). Acute kidney injury at presentation was defined by an increase in serum creatinine by ≥0.3 mg/dL in the first 48 hours, and hypotension was defined by a sustained systolic blood pressure less than 90 mmHg and diastolic less than 60 mmHg (14).
Statistical analysis
Predictive analytic software (PASW) was utilized for statistical analysis. All data were expressed as mean ± standard error (SE). Frequencies of data were presented as number of patients and percentage. Continuous variables between the dabigatran group and the warfarin group were compared by using Student’s t-test. Chi-square was used to compare dichotomous variables between the two groups. Stepwise multiple regression analysis was performed to adjust for confounding variables that might affect number of PRBC transfusions including history of CKD, presence of hypotension, and level of hemoglobin at presentation. P values <0.05 were considered significant.
Results
During the study period, 16 patients taking dabigatran and 26 patients taking warfarin were admitted were admitted to St. John Hospital and Medical Center due to GI bleeding during 2009 to 2012. Three patients in the warfarin group were excluded from the study including one patient with history of active alcohol ingestion, one patient with cirrhosis, and a patient with platelet count lower than 50,000/microliter. Since dabigatran became commercially available (October 2011), it accounted for 1.7% of all GI bleeding admission at our institute (16/921). Ultimately, 13 patients in dabigatran group and 26 patients in the warfarin group were included in the study. Demographic data, previous GI pathology, past medical history, active medications, and baseline laboratory results of both groups are shown in Table 1. Mean age of patients in both groups was similar (77.9±10 years for the dabigatran group vs. 76±10.2 years for the warfarin group, P=0.59). Sex and race between the two groups were not different. Mean BMI in the dabigatran group was slightly higher than the warfarin group, but the P value was not significant (34.9±14.4 vs. 30±7.5, P=0.26). There were no statistical significances between the two groups for any demographic data, history of previous GI pathology, clinical parameter, medication use except history of aspirin use, which was significantly higher in the dabigatran group (85% vs. 50%, P=0.036). Five patients in the dabigatran group had CKD stage 3 (38.5%), and one patient had CKD stage 4. In the warfarin group, ten patients had CKD stage 3 (38.5%), and a patient had CKD stage 4. The rest of the patients had CrCl >60 mL/min (53.8% in the dabigatran group and 57.7% in the warfarin group). Mean CrCl was almost identical in both groups (64±24.5 mL/min in the dabigatran group and 64.9±23.8 mL/min in the warfarin group). Baseline hemoglobin level, platelet count, and creatinine level were not found to be different between the two groups.
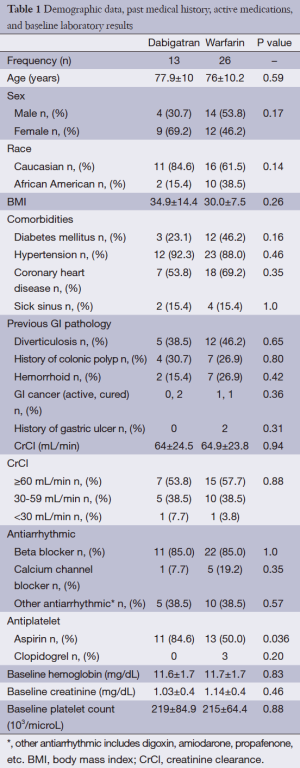
Full table
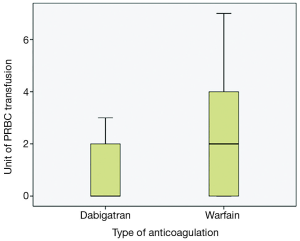
The majority of the patients in both groups presented with lower GI bleeding, followed by symptomatic anemia and upper GI bleeding; however, there were no statistical differences between both groups (Table 2). Trend of hemoglobin in both groups are shown in Figures 1 and 2. First hemoglobin, platelet count at presentation, creatinine level, and INR level between two the groups are shown Table 2. First hemoglobin in the dabigatran group was found to be slightly higher than the warfarin group, but the P value was not significant (P=0.34). At presentation, platelet count was significantly lower in the dabigatran group (189±60 vs. 240±89 103/dL, P=0.045). Mean INR level of the dabigatran group was 1.81±0.9 and mean INR of the warfarin group was 2.54±0.3. None of the patients in the dabigatran group received FFP while 10 patients in the warfarin group received FFP. Creatinine levels at presentation in both groups were almost identical (1.35±1 vs. 1.35±0.8 mg/dL, P=0.99). Out of 13 patients in the dabigatran group, only seven cases had aPTT checked at presentation (54%). Five of them had high aPTT levels while two patients had normal aPTT levels. Clinical outcomes including hypotension, tachycardia, acute kidney injury, length of stay, ICU admission, and death between the two groups are also shown in Table 2. Hypotension was found in 7.7% of the dabigatran group compared with 30.8% in the warfarin group (P=0.11). The presence of tachycardia at presentation, acute kidney injury, length of stay, ICU admission, and death were not different between two groups. There was significantly fewer units of PRBC transfusions in the dabigatran group compared with the warfarin group (0.69±1.1 vs. 1.92±2.2 units, P=0.024) (Figure 3). After multivariate analysis was performed adjusting for history of CKD, hemoglobin level at presentation, and presence of hypotension, there was significant association between hemoglobin at presentation (b =–4.8, P<0.01), type of anticoagulation (b =1.013, P=0.043), and the quantity of transfused PRBCs. The regression analysis also revealed higher quantity of PRBC transfusions in the warfarin group after controlling for initial hemoglobin at presentation and history of CKD.
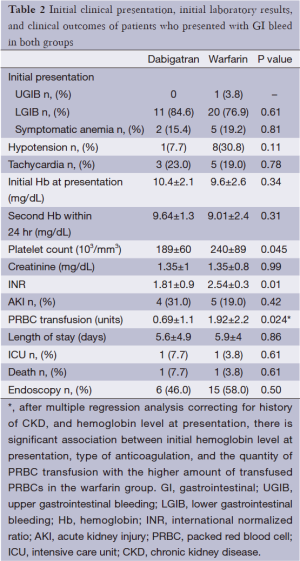
Full table
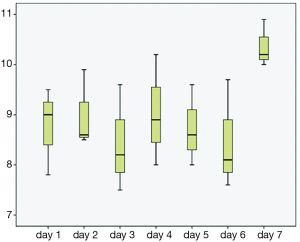
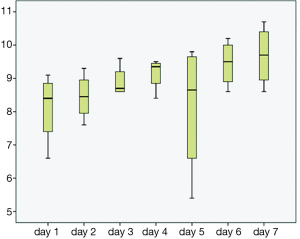
Conclusions
In this present study, although the patients with GI bleeding who had therapeutic INR level received more PRBC transfusions compared with the dabigatran group (1.92±2.2 vs. 0.69±1.1 units, P=0.024); the clinical outcomes including hypotension, tachycardia, acute kidney injury, length of stay, ICU admission, and death were not significantly different between the two groups. Moreover, the mortality rates of both groups were similar (7.7% in the dabigatran group and 3.8% in the warfarin group, P=0.61).
Dabigatran has several advantages over warfarin in terms of pharmacokinetic and pharmacodynamic properties. After ingestion, dabigatran is hydrolyzed by liver into an active form and reaches peak concentration rapidly within 1 to 2 hours (2). Moreover, unlike warfarin, moderate hepatic impairment (Child Pugh score of B or less) does not affect the concentration of dabigatran; therefore, dose adjustment in this circumstance is not required (15). In the serum, only 35% of dabigatran binds to plasma protein compared with 99% of warfarin (1). This resulted in significantly lower risk of drug interaction with dabigatran compared with warfarin. In healthy individuals, dabigatran is mainly cleared unchanged by kidney and the half-life was found to be ranging from 12 to 17 hours (16). The anticoagulation effect of dabigatran is directly correlated with the level of the medication and, therefore, monitoring test is unnecessary (1). Additionally, the RE-LY trial demonstrated that the effectiveness of dabigatran was comparable to warfarin in terms of stroke prevention and had lower risk of total bleeding events, intracranial bleeding, and life-threatening bleeding (3). For these reasons, utilization of dabigatran is expected to increase in the near future (17).
Due to the recent introduction of dabigatran, main enigmas physicians are currently facing are the significant risk of GI bleeding of dabigatran and lack of experience of physicians in the management of GI bleeding from dabigatran. In the RE-LY trial, Connolly et al. reported risk of GI bleeding of dabigatran to be significantly higher than warfarin (3). A meta-analysis of randomized controlled trials on the risk of bleeding with dabigatran also confirmed this finding (18). Several published cases of severe and fatal GI bleeding resulted from dabigatran have been occasionally reported as well (4-7). The use of dabigatran among patients with renal impairment also remains a topic for debate considering the FDA has approved the use of 75-mg of dabigatran for patients with CrCl of 15 to 30 mL/min without being evaluated in a randomized controlled trial and, additionally, patients with CrCl <30 mL/min were not included in the RE-LY trial (3). Moreover, the half-life of dabigatran can be prolonged to 18 hours in mild to moderate renal impairment and up to 28 hours in severe renal impairment (16). Most importantly, no accurate laboratory monitoring and reverse agent are commercially available. Hence, the management of GI bleeding in this situation has become a major concern among physicians, especially in patients with acute renal impairment or CKD.
Severity and outcomes of patients with GI bleeding from dabigatran, however, are not well studied and most data is very limited to case reports and series. Kernan et al. reported a case of fatal GI hemorrhage after just one single dose of dabigatran in a 92-year-old man with CrCl <30 mL/min (5). A case of hemorrhagic gastritis from dabigatran in a patient with chronic renal impairment has also been reported (6). Ross et al. reported four cases of severe GI bleeding from dabigatran and found no death among these patients (9). Yet, given reported cases of severe GI bleeding from dabigatran and unavailability of accurate monitoring and reversal agents, our study showed comparable clinical outcomes and length of stay in predominately elderly individuals (mean age of 77.9±10 years) with CrCl >30 mL/min who were hospitalized for GI bleeding from dabigatran compared with warfarin, even though the quantity of PRBC transfusions was higher in the warfarin group.
In our study, none of the patients in the dabigatran group received FFP. This implies that physicians were aware that there is no benefit of FFP in patients with GI bleeding caused by dabigatran. In contrast, the awareness of aPTT as a recommended measurement of dabigatran activity was low considering only 54% of patients (7/13) in the dabigatran group had aPTT checked during the hospitalization.
Currently, patients with GI bleeding from dabigatran are primarily managed conservatively. Recently, American Heart Association (AHA) has published recommendations regarding management and approach to bleeding in patients taking dabigatran (10). In general, treatment of patients with GI bleeding from dabigatran should be individualized according to location of bleeding, severity of bleeding, the probability of overdose, and kidney function. During the bleeding episode, in all cases, dabigatran should be discontinued, and aPTT and TT should be measured to affirm the physiologic effect of dabigatran in the body. Although, aPTT and TT are sensitive measurements for detecting the presence of dabigatran, aPTT and TT cease to increase once the level of dabigatran is beyond the therapeutic range (19). Therefore, they are not appropriate for detecting overdose of dabigatran. PT and INR, on the opposite side, are insensitive to the effect of dabigatran, and should not be utilized to evaluate the effect of dabigatran (2,19). Hemoclot and ecarin clotting time are the gold standard measurements and have linear relationship with plasma level of dabigatran; however, they are not commercially available (19,20). Serum creatinine and baseline creatinine are also crucially important in assessing renal filtration function in order to estimate the half-life of dabigatran especially in moderate to severe renal impairment (16). Additionally, last dose of dabigatran should be determined to anticipate the duration of anticoagulation effect of dabigatran. If a patient presents within four hours after the last dose of dabigatran or overdose is suspected, activated charcoal should be immediately considered (21). In case of minor GI bleeding, holding dabigatran for 1 to 2 doses until bleeding stops is often sufficient since concentration of dabigatran drops quickly after discontinuation (20). For moderate to severe GI bleeding, dabigatran should be immediately stopped along with other antiplatelet agents. Appropriate fluid resuscitation, cross matching for blood products, and prompt endoscopic evaluation should be prepared. Most of the time, patients can be successfully managed conservatively by watchful waiting, control of bleeding site, fluid resuscitation, and appropriate transfusion without the use of reversal agents. However, for life threatening GI bleeding and moderate GI bleeding with unsuccessful conservative treatment, non-specific reversal agents such as, factor eight inhibitor bypassing activity (FEIBA), recombinant activated factor VII (rFVIIa), or FFP should be considered even though data regarding their efficacy is limited. Despite prothrombin complex concentration (PCC) was recently approved for reversal of the effect of warfarin and a study in an animal model demonstrated the effectiveness of PCC in reversing bleeding from dabigatran, a study in healthy volunteers showed that PCC failed to reverse aPTT prolongation from dabigatran (9,22). The data regarding reversal of dabigatran by using rFVIIa and FFP is very limited and mainly obtained from reported cases (23-26). Hemodialysis has been shown to be effective for removing dabigatran from the body; however, rebound of dabigatran level after hemodialysis has been observed due to large volume of distribution (27-31). Thus, hemodialysis should be considered as a last resort when other methods fail to stop bleeding, and prolonged session of hemodialysis for more than four hours has been suggested to prevent rebound of dabigatran level (28). Without any proven reversal agents, the great hope has been putting in the development of antibody to dabigatran which is currently under investigation (32).
Our study has all the limitations of small case series. We were unable to control for the clinical decision-making in the management of GI bleeding or other factors, which influenced the original choice of anticoagulation. We did not have a protocol in place for in vitro diagnostics for GI bleeding on novel anticoagulants. Other limitation in this study also included the higher prevalence of concurrent aspirin use in the dabigatran group (84.6% vs. 50%, P=0.036) According to the PETRO study, the incidence of major bleeding and clinical relevant bleeding were not different between patients taking dabigatran 150 mg twice a day plus aspirin and those taking dabigatran alone (33). Therefore, the history of concurrent aspirin use in this study might not affect the incidence and outcome of GI bleeding. The subgroup analysis of the RE-LY trial looking specifically in patients with GI bleeding will provide very useful information regarding the outcome of GI bleeding in patients taking dabigatran.
In summary, clinical outcomes in patients taking dabigatran who present to the hospital for GI bleeding are similar to those taking warfarin an INR in the therapeutic range. Given the mean CrCl of patients in the dabigatran group was 64±24.5 mL/min and only one patient in the dabigatran group had CrCl <30 mL/min, the outcomes of patients in the dabigatran group in this study should, therefore, be applied strictly to elderly patients who have CrCl ≥30 mL/min. The short half-life of dabigatran appears to be the major factor that limits bleeding complications on this novel agent.
Acknowledgements
The authors gratefully appreciated the support and assistant from Susana Szpunar, PhD and the research department of St John Hospital and Medical Center. We also would like to specially thank Peter McCullough, MD for supervising the study and provide valuable suggestions.
Disclosure: The authors declare no conflict of interest.
References
- Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet 2008;47:285-95. [PubMed]
- Stangier J, Rathgen K, Stähle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292-303. [PubMed]
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [PubMed]
- Wychowski MK, Kouides PA. Dabigatran-induced gastrointestinal bleeding in an elderly patient with moderate renal impairment. Ann Pharmacother 2012;46:e10. [PubMed]
- Kernan L, Ito S, Shirazi F, et al. Fatal gastrointestinal hemorrhage after a single dose of dabigatran. Clin Toxicol (Phila) 2012;50:571-3. [PubMed]
- Fellows SE, Rosini JM, Curtis JA, et al. Hemorrhagic gastritis with dabigatran in a patient with renal insufficiency. J Emerg Med 2013;44:e221-5. [PubMed]
- Holm J, Taskiran M. Dabigatran as cause of severe gastrointestinal bleeding in a Jehovah's Witness patient. Ugeskr Laeger 2013;175:334-6. [PubMed]
- Legrand M, Mateo J, Aribaud A, et al. The use of dabigatran in elderly patients. Arch Intern Med 2011;171:1285-6. [PubMed]
- Ross B, Miller MA, Ditch K, et al. Clinical experience of life-threatening dabigatran-related bleeding at a large, tertiary care, academic medical center: a case series. J Med Toxicol 2014;10:223-8. [PubMed]
- Weitz JI, Quinlan DJ, Eikelboom JW. Periprocedural management and approach to bleeding in patients taking dabigatran. Circulation 2012;126:2428-32. [PubMed]
- Kilbride HS, Stevens PE, Eaglestone G, et al. Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis 2013;61:57-66. [PubMed]
- Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012;307:1941-51. [PubMed]
- Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137-47. [PubMed]
- Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 2013;61:649-72. [PubMed]
- Stangier J, Stähle H, Rathgen K, et al. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairment. J Clin Pharmacol 2008;48:1411-9. [PubMed]
- Stangier J, Rathgen K, Stähle H, et al. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010;49:259-68. [PubMed]
- Wartak SA, Bartholomew JR. Dabigatran: will it change clinical practice? Cleve Clin J Med 2011;78:657-64. [PubMed]
- Bloom BJ, Filion KB, Atallah R, et al. Meta-analysis of randomized controlled trials on the risk of bleeding with dabigatran. Am J Cardiol 2014;113:1066-74. [PubMed]
- Douxfils J, Mullier F, Robert S, et al. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost 2012;107:985-97. [PubMed]
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate--a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010;103:1116-27. [PubMed]
- van Ryn J, Neubauer M, Flieg R, et al. Successful removal of dabigatran in flowing blood with an activated charcoal hemoperfusion column in an in vitro test system. Pathophysiol Haemost Thromb 2010;37:A94.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573-9. [PubMed]
- Warkentin TE, Margetts P, Connolly SJ, et al. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood 2012;119:2172-4. [PubMed]
- Dumkow LE, Voss JR, Peters M, et al. Reversal of dabigatran-induced bleeding with a prothrombin complex concentrate and fresh frozen plasma. Am J Health Syst Pharm 2012;69:1646-50. [PubMed]
- Htun KT, McFadyen J, Tran HA. The successful management of dabigatran-associated critical end-organ bleeding with recombinant factor VIIa. Ann Hematol 2014. [Epub ahead of print]. [PubMed]
- van Ryn J, Ruehl D, Priepke H, et al. Reversibility of the anticoagulant effect of high doses of the direct thrombin inhibitor dabigatran, by recombinant factor VIIa or activated prothrombin complex concentrate. Haematologica 2008;93:148.
- Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 2013;109:596-605. [PubMed]
- Chang DN, Dager WE, Chin AI. Removal of dabigatran by hemodialysis. Am J Kidney Dis 2013;61:487-9. [PubMed]
- Chen BC, Sheth NR, Dadzie KA, et al. Hemodialysis for the treatment of pulmonary hemorrhage from dabigatran overdose. Am J Kidney Dis 2013;62:591-4. [PubMed]
- Liesenfeld KH, Staab A, Härtter S, et al. Pharmacometric characterization of dabigatran hemodialysis. Clin Pharmacokinet 2013;52:453-62. [PubMed]
- Esnault P, Gaillard PE, Cotte J, et al. Haemodialysis before emergency surgery in a patient treated with dabigatran. Br J Anaesth 2013;111:776-7. [PubMed]
- Van Ryn J, Litzenburger T, Waterman A. eds. An antibody selective to dabigatran safely neutralizes both dabigatran-induced anticoagulant and bleeding activity in in vitro and in vivo models. J Thromb Haemost 2011;9:Suppl 2.
- Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol 2007;100:1419-26. [PubMed]


