
Mineralocorticoid receptor blockade improves pulmonary hypertension and right ventricular function in bronchopulmonary dysplasia: a case report
Introduction
Bronchopulmonary dysplasia (BPD) is a combined pulmonary vascular and parenchymal lung disease representing the most common cause of chronic lung disease (CLD) in infancy (1,2). For almost two decades BPD has been defined as the need for supplemental oxygen use either at a corrected age of 36 gestational weeks (p.m.) or after 28 days of life (3), and the severity of BPD (mild, moderate or severe) has been determined at 36 weeks post-menstrual age (PMA) (3). However, in 2019, a new definition of BPD was proposed that includes infants on any form of respiratory support (such as high-flow nasal cannula) at 36 corrected weeks (p.m.), even in the absence of any supplemental oxygen administration (4). Regardless of which BPD definition may be used, pulmonary hypertension (PH)/pulmonary hypertensive vascular disease (PHVD) might be evident even before the formal diagnosis of BPD can be made (5), and is—importantly—a major determinant of clinical outcome in BPD: approximately 25% of infants with moderate to severe BPD develop PH (6,7) that affects heart and lungs, thereby greatly increasing mortality (i.e., 47% of BPD-infants die 2 years after diagnosis of PH) (8-10).
We present the following case in accordance with the CARE Guideline (available at http://dx.doi.org/10.21037/cdt.2020.02.05).
Case presentation
A male, preterm infant [28 2/7 PMA; birth weight 600 g] presented postnatally with respiratory distress syndrome (RDS). On the second day of postnatal life the infant suffered from acute pulmonary hemorrhage so that the infant was transitioned from conventional to high frequency oscillatory ventilation (HFOV). Echocardiogram at this point showed adequate systolic function of the right ventricle (RV), but the degree of PH was estimated to be nearly systemic arterial pressure, based on the full flattening of the interventricular septum in end systole, in the absence of tricuspid regurgitation (TR). In total, the infant underwent 12 postnatal days on mechanical ventilation, 40 days on continuous positive airway pressure (CPAP), 130 in hospital days, and 127 days on supplemental oxygen.
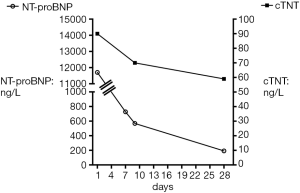
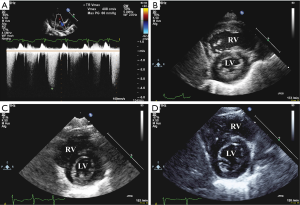
Four months later, at 44 gestational + postnatal weeks p.m. (body weight 2,600 g), the patient was spontaneously breathing on 1 L/min supplemental oxygen via nasal cannula, had significant tachypnea and dyspnea, negative viral panel, and patchy hyperdensities on chest X-ray, consistent with BPD. Laboratory findings of elevated NT-pro-BNP (11,702 ng/L) and cardiac troponin T (cTNT; 90 ng/L; reference values: NT-pro-BNP: ≤84 ng/L; cTNT: ≤14 ng/L; Figure 1) indicated heart failure and moderate myocardial ischemia. Echocardiographically, RV systolic pressure (RVSP) was estimated to be systemic or even slightly supra-systemic, using the CW Doppler velocity of the TR jet (4.1 m/s) and the simplified Bernoulli equation (Figure 2A) . The RV was dilated and hypertrophied (Figure 2B) and longitudinal systolic RV function as judged by tricuspid annular peak systolic excursion (TAPSE), was moderately decreased (TAPSE 0.61 cm; reference values: 0.68–1.15 cm for term infants 0–30 days). There was mild TR (I°). Systolic left ventricular (LV) function and renal function were normal.
Treatment and outcome
To decrease pulmonary artery pressure and improve RV function, we initiated combined treatment with the mineralocorticoid receptor (MR) blocker spironolactone and hydrochlorothiazide (HCTZ; both 2×3 mg p.o.). After 7 days, the infant’s tachypnea and dyspnea disappeared and general clinical condition had greatly improved, so that the patient was discharged from the hospital on supplemental oxygen (0.1 L/min, SpO2 ≥93%) 12 days after spironolactone/HCTZ had been started.
Intriguingly, NT-pro-BNP and cTNT decreased substantially within the first 1–4 weeks of MR blockade with spironolactone (Figure 1). Consistent with the laboratory findings, echocardiography 4 weeks after the start of MR blockade confirmed much improved PH and decreased RV dilation: TR was trivial and RVSP was measured to be less than half-systemic. The right ventricular internal diameter in diastole (RVIDD) decreased from 1.21 to 1.05 cm. RV/LV systolic function and diameter were normal (Figure 2C). Because of these rapid improvements, a planned cardiac catheterization was cancelled and no additional “PH specific” therapy (e.g., sildenafil, bosentan) was initiated so far (5). Three months after start of spironolactone and HCTZ the patient had been weaned off oxygen, had less than half-systemic RV systolic pressure, decreased RVIDD (0.86 cm), decreased serum NT-pro-BNP (124 ng/L), and cTNT (24 ng/L) and normal longitudinal systolic RV function [TAPSE 1.1 cm; reference values 0.85–1.42 cm for term infants 1–3 months old (11)] (Figure 1,2D). After 9 months of MR blockade at a follow-up visit, that patient had constantly low (normal) serum NT-pro-BNP (129 ng/L) and serum cTNT that was further decreased into the normal reference range (<10 ng/L) (Figure 1). In accordance with these laboratory and imaging findings, tachydyspnea and intermittent cyanosis disappeared within the first week of spironolactone/HCTZ treatment.
Discussion
BPD and associated PH/RV dysfunction in the first year of life are life-threatening conditions awaiting innovative diagnostic biomarkers such as galectin-3 (12), and effective treatment strategies, including MR blocking agents (13-16) and cell-based therapies that have the potential to reverse the underlying CLD (17-20).
We cannot fully rule out a synergistic effect of HCTZ on MR blockade with spironolactone in this patient. However, the effect of the add-on medication on PH, biomarkers, and clinical status was rapid (within days) while the diuretic effect of both HCTZ and spironolactone is known to be mild and slow. Thus, we suggest MR blockade with spironolactone could be an adjuvant treatment for PH and RV dilation/dysfunction in infants. Clinically, both eplerenone and spironolactone are frequently used in patients with ventricular hypertrophy/remodeling and in those with RV and/or LV diastolic dysfunction [precapillary PH/pulmonary arterial hypertension (PAH), or heart failure with preserved ejection fraction (HFpEF) with or without postcapillary PH].
In a meta-analysis of nine HFpEF trials MR antagonist treatment significantly improved indices of cardiac structure and function, suggesting a decrease in LV filling pressure and reverse cardiac remodeling (21). The current NIH-sponsored “Pilot Study of Effect of Spironolactone Therapy on Exercise Capacity and Endothelial Dysfunction in Pulmonary Arterial Hypertension” (NCT0171262) aims to enroll 70 adult group 1 patients to either spironolactone (25–50 mg) or placebo. Patients with idiopathic PAH (IPAH) and disease-associated PAH will be recruited and enrolled in a randomized, double blinded, placebo-controlled study of early treatment with spironolactone to investigate its effects on exercise capacity, clinical worsening, and vascular inflammation in vivo.
Taken together, preterm infants with BPD and persistent, more than mild PH (>1/2 systemic RV pressure), and those with “out of proportion PH” (mild BPD/CLD but severe PH), should strongly be considered for early, full diagnostic work-up (including cardiac catheterization and subsequent “PH-targeted” pharmacotherapy (e.g., sildenafil +/− endothelin receptor antagonist) (5). Treatment efficacy of spironolactone and HCTZ in preterm BPD infants with right ventricular hypertrophy (RVH) and PH should be interpreted with caution as the current number of the published observations (including our case) is small, and more specific, prospective pediatric and adult PH studies on the efficacy of (add-on) MR blockade are warranted. Nevertheless, the 2019 Updated Guidelines of the European Pediatric Pulmonary Vascular Disease Network (EPPVDN) in this respect recommend “In infants with severe BPD with or without PH, judicious fluid management is important, and may include treatment with diuretics (i.e., HCTZ and spironolactone), as long as cardiac pre-load is adequate” (class of recommendation IA, level of evidence B) (5).
Acknowledgments
Funding: G Hansmann receives funding from the German Research Foundation (DFG; HA4348/2-2, HA4348/6-2 KFO311).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Martin Koestenberger, Harm-Jan Bogaard and Georg Hansmann) for the series “Right Ventricular Dysfunction” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Editor-in-Chief and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt.2020.02.05). The series “Right Ventricular Dysfunction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thebaud B, Goss KN, Laughon M, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers 2019;5:78. [Crossref] [PubMed]
- Stoll BJ, Hansen NI, Bell EF, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA 2015;314:1039-51. [Crossref] [PubMed]
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723-9. [Crossref] [PubMed]
- Jensen EA, Dysart K, Gantz MG, et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am J Respir Crit Care Med 2019;200:751-9. [Crossref] [PubMed]
- Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant 2019;38:879-901. [Crossref] [PubMed]
- Weismann CG, Asnes JD, Bazzy-Asaad A, et al. Pulmonary hypertension in preterm infants: results of a prospective screening program. J Perinatol 2017;37:572-7. [Crossref] [PubMed]
- Mourani PM, Sontag MK, Younoszai A, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med 2015;191:87-95. [Crossref] [PubMed]
- Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007;120:1260-9. [Crossref] [PubMed]
- Altit G, Bhombal S, Feinstein J, et al. Diminished right ventricular function at diagnosis of pulmonary hypertension is associated with mortality in bronchopulmonary dysplasia. Pulm Circ 2019;9:2045894019878598. [Crossref] [PubMed]
- Mirza H, Ziegler J, Ford S, et al. Pulmonary hypertension in preterm infants: prevalence and association with bronchopulmonary dysplasia. J Pediatr 2014;165:909-14.e1. [PubMed]
- Koestenberger M, Friedberg MK, Ravekes W, et al. Non-Invasive Imaging for Congenital Heart Disease: Recent Innovations in Transthoracic Echocardiography. J Clin Exp Cardiolog 2012.Suppl 8:2. [PubMed]
- Calvier L, Legchenko E, Grimm L, et al. Galectin-3 and aldosterone as potential tandem biomarkers in pulmonary arterial hypertension. Heart 2016;102:390-6. [Crossref] [PubMed]
- Preston IR, Sagliani KD, Warburton RR, et al. Mineralocorticoid receptor antagonism attenuates experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2013;304:L678-88. [Crossref] [PubMed]
- Stewart A, Brion LP, Ambrosio-Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev 2011.CD001817. [Crossref] [PubMed]
- Borlaug BA. Heart failure: Aldosterone antagonism for HFpEF. Nat Rev Cardiol 2013;10:244-6. [Crossref] [PubMed]
- Maron BA, Waxman AB, Opotowsky AR, et al. Effectiveness of spironolactone plus ambrisentan for treatment of pulmonary arterial hypertension (from the [ARIES] study 1 and 2 trials). Am J Cardiol 2013;112:720-5. [Crossref] [PubMed]
- Hansmann G, Fernandez-Gonzalez A, Aslam M, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ 2012;2:170-81. [Crossref] [PubMed]
- Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr 2014;164:966-972.e6. [Crossref] [PubMed]
- Alphonse RS, Vadivel A, Fung M, et al. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth. Circulation 2014;129:2144-57. [Crossref] [PubMed]
- Willis GR, Fernandez-Gonzalez A, Anastas J, et al. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med 2018;197:104-16. [Crossref] [PubMed]
- Kapelios CJ, Murrow JR, Nuhrenberg TG, et al. Effect of mineralocorticoid receptor antagonists on cardiac function in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis of randomized controlled trials. Heart Fail Rev 2019;24:367-77. [Crossref] [PubMed]


