Traumatic aortic injury: CT findings, mimics, and therapeutic options
Traumatic aortic injury (TAI) is a life threatening condition that requires prompt diagnosis and management. It is estimated that aortic injuries are lethal in 80-90% of cases (1). With the improved in-field emergency care available today more patients survive to obtain diagnostic imaging and treatment. Computed tomographic angiography (CTA) is the current method of choice for assessing traumatic injuries as it is fast and readily available in nearly all emergency departments. CTA is also very accurate for diagnosis of aortic injury. In one surgical series all cases of TAI were appropriately diagnosed by computed tomography (CT) (2).
Undiagnosed and untreated TAI has a very high mortality of 90% at 4 months (1). The relative infrequency of TAIs coupled with their high mortality rate makes it imperative that radiologists are familiar with the CT findings of TAI.
Proposed injury mechanisms
The mechanism of TAI has not been completely determined despite extensive research. Proposed mechanisms include: rapid deceleration, shearing forces, osseous pinch, and hydrostatic forces or water-hammer phenomenon. Rapid deceleration in the lateral direction, such as in side impact motor vehicle collisions, and in the antero-posterior direction, such as in head on collisions, causes shifting of the mediastinal structures sufficient to create shear stress on the aorta at the areas it is relatively immobile (3). The osseous pinch mechanism was proposed by Crass et al. who used an animal model to demonstrate their proposed TAI mechanism (4). The osseous pinch is due to compression of the aorta between the spine and manubrium, first rib, and/or medial clavicles. This mechanistic theory was also supported by a subsequent study that analyzed CT scans of 22 patients with surgically or angiographically proven TAI and used the osseous pinch mechanism to accurately predict the location of the aortic injury in all 22 patients (5). The water-hammer effect is caused by a sudden increase in intrathoracic pressure that can result in tears at the aortic isthmus or aortic root injury (6). TAIs are likely due to some combination of the above mechanisms as no dominant mechanism has yet been found.
Injury locations & associated injuries
TAIs most commonly occur at sites of aortic tethering; the aortic root, the isthmus and at the diaphragmatic hiatus. Injuries at the isthmus and abdominal aorta are the most commonly seen in surgical series and on imaging series as injuries at the other locations are typically fatal. In one surgical series (7) 95% of patients had injuries at the aortic isthmus compared with autopsy series that report only 45-58% of injuries to be at the isthmus (1,8). The differences in the reported prevalence of injury locations are likely due to the differences in mortality.
Thoracic TAIs are associated with a numerous additional injuries. The most common associated injuries are multiple rib fractures which were seen in 75% of patients in one autopsy series (9). Sternal and first rib fractures are also fairly common in patients with TAI reflecting the significant force required to cause these injuries. Injuries to the heart, spleen and liver are also frequently seen in conjunction with aortic injury.
In the abdomen the most common site of injury is the infrarenal abdominal aorta. These injuries are typically related to lap belt compression. There are a number of additional abdominal injuries that are associated with TAI including lumbar spine fracture, splenic injury, pelvic fractures, bowel injury and solid organ injury. Lumbar spine fracture has the highest association with traumatic abdominal aortic injury (10) and if seen should prompt a careful evaluation of the aorta and vice versa.
Typical imaging findings
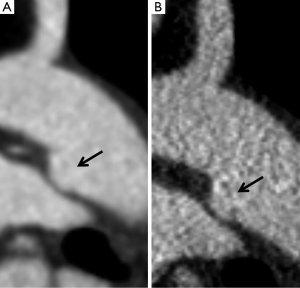
The CT findings of TAI can be divided into direct signs of injury and indirect or associated findings. Direct findings of aortic injury include intramural hematoma, intimal flap and pseudoaneurysm (Figure 1). Injuries that only involve the intima, classified as minimal aortic injuries, should only have direct findings of TAI. Minimal aortic injuries can present with an intimal flap, intraluminal aortic thrombus or intramural hematoma. With the improvement in technology allowing thinner CT slice thickness minimal aortic injuries are being diagnosed more frequently (Figure 2).
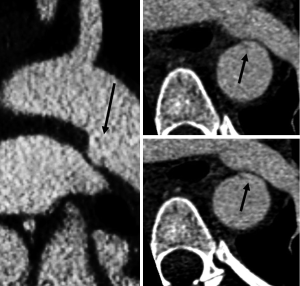
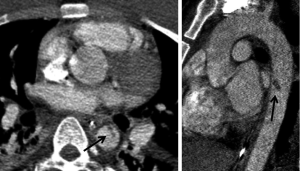
More severe injuries often have both direct and indirect findings of TAI. Indirect signs include periaortic hematoma, change in aortic caliber and irregular aortic contour (Figure 3). Changes in aortic caliber can be subtle on axial imaging and are often easier to appreciate with multiplanar reformatted images (Figure 4).
Chronic pseudoaneurysms can develop at the sites of undiagnosed and/or untreated aortic injury. These often have extensive peripheral calcification (Figure 5) and may also contain thrombus. The peripheral calcification is thought to be protective against aneurysm rupture (11).
Mimics and pitfalls
There are multiple normal anatomic variants and conditions that can mimic an acute TAI as well as a number of technical pitfalls that can make diagnosis more difficult. It is important to be aware of these potential confounders to assure accurate diagnosis and subsequent appropriate therapy for patients with TAIs.
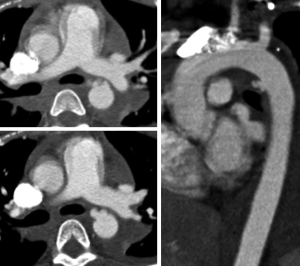
Ductal remnants, a diverticulum or small bump, are normal remnants of the embryologic ductus arteriosus. This normal variant can simulate injury and can be very perplexing for the inexperienced or unaware radiologist. The ductal diverticulum is a remnant of the closed or partially closed ductus arteriosus which connects the pulmonary artery to the aorta in fetal circulation. Ductal remnants are located at the inferior surface of the aortic arch near the aortic isthmus which leads to their confusion with TAIs (Figure 6). Ductal remnants are typically smooth walled and have obtuse margins that are continuous with the aortic wall and are often calcified (12). The presence of calcification can be very helpful in distinguishing a ductal remnant from a TAI with the presence of calcification favoring a benign ductal remnant.

Infundibula can have a similar appearance to a ductal remnant, but are found at the origin of bronchial or intercostal arteries. They can also be confused with a small pseudoaneurysm. Infundibula are typically cone shaped and smooth walled with a small artery extending from the apex (Figure 7). The small artery extending from an infundibulum can be difficult to definitively distinguish with CT and can be confirmed with angiography.
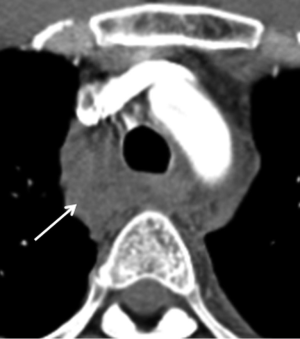
Mediastinal hematoma in conjunction with a ductal remnant or infundibulum can create further confusion. Mediastinal hematomas can be due to injury to other structures including the pulmonary artery, great vessels or mediastinal veins, or even fractures of vertebral bodies. Presence of a mediastinal hematoma should prompt a careful search for an aortic, pulmonary artery or great vessel injury. In the absence of an identified arterial injury the hematoma is likely venous. A preserved fat plane around the aorta or hematoma centered away from the aorta is less likely to be associated with aortic injury and more likely to be venous (Figure 8).
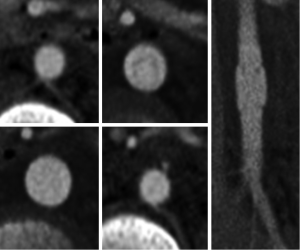
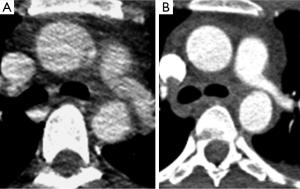
In addition to anatomic mimics there are a number of technical pitfalls to be aware of including motion artifact and inappropriate slice thickness selection. Motion artifact can be due to respiratory or cardiac motion. On non-gated CT studies there is often artifact at the aortic root due to cardiac motion and pulsation of the aorta. This artifact can very closely resemble an aortic dissection (13). Motion artifact can be worsened in trauma patients who are often hypotensive and tachycardic which can result in hyperdynamic cardiac movement. The best way to distinguish a true aortic root injury from motion artifact is to repeat thoracic imaging with ECG gating; and echocardiography can be a reasonable alternative. The difference between a study done without and with ECG gating is illustrated in (Figure 9). In our institution all of the chest CT done as part of a trauma survey are acquired without ECG gating. Since the majority of TAIs are at the aortic isthmus, which is typically well seen on non-gated studies, we feel the additional radiation exposure and time required for setup and acquisition of an ECG gated study is not necessary for every patient. On the rare occasion that there is a question regarding the aortic root we will repeat the chest CT with retrospective ECG gating. This allows for reconstruction at any phase of the cardiac cycle which can be helpful in problem solving.
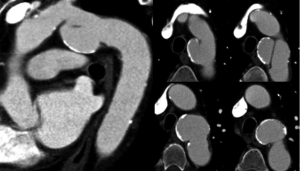
As part of the standard trauma protocol at our institution coronal and sagittal reconstructions with 2 mm slice thickness are performed for every exam. In some instances making reconstructions with a 1 mm slice thickness and/or sharper kernel may be useful for problem solving. In addition, if the scan is ECG-gated in retrospective fashion, some phases of the cardiac cycle may demonstrate an injury better than others. For example, Figure 10 demonstrates a 22-year-old male involved in a driver’s side impact motor vehicle collision. On the initial 2 mm reconstructions there was a suggestion of an intimal flap in the aortic arch with a small pseudoaneurysm. Additional 1 mm slice thickness reconstructions were obtained and confirmed the TAI by demonstrating it more convincingly. Always consider additional reconstructions with a different slice thickness, different imaging plane, different reconstruction kernel, or different phase of the cardiac cycle to help clarify ambiguous findings.
Treatment options
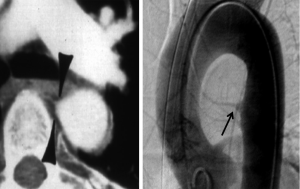
Treatment options for TAI can be divided into three categories: open surgical repair, endovascular repair and medical management. Traditionally TAI has been treated with open surgical repair and this is still the procedure of choice to repair injuries involving the aortic root, ascending aorta and aortic arch. Open surgical repair may also still be the preferred option for repair of isthmic aortic injuries in stable or young patients depending on the preference and experience of the surgical team at each center. Open surgical repair is typically done with an interposition graft that is slightly higher in attenuation than the native aorta and will have a slight contour change at both the proximal and distal anastomoses (Figure 11A). Kinks may be seen within the graft material that are usually of no clinical consequence. Felt pledgets can be used to reinforce the anastomoses or to repair aortic cannulation sites. Felt pledges are high attenuation and can be confused with a small pseudoaneurysm if the interpreting physician is not familiar with the appearance. Precontrast images can be helpful in identifying felt pledgets. Although imaging both prior to and after contrast administration increases radiation dose, a single precontrast phase obtained on the first postoperative scan can establish a useful baseline that can be referenced on serial followup single-phase imaging.
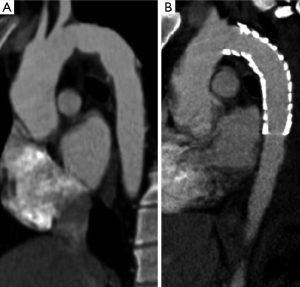
Endovascular repair of TAI has become the initial procedure of choice in some centers for management of TAI (Figure 11B) involving the aortic isthmus, descending thoracic or abdominal aorta. A prospective multicenter study by the American Association for the Surgery of Trauma demonstrated lower mortality in the endovascular repair group than in the open repair group (7.2% vs. 23.5%) and fewer blood transfusions in the endovascular repair group (14). Other advantages of endovascular repair include lower risk of paraplegia and no need to open the chest cavity. However, endovascular repair requires regular imaging follow up to assess for complications such as graft migration, graft fracture, endoleak, graft infection or access site complications, and long-term outcomes have not been established. The need for serial imaging follow up and the higher incidence of post procedural complications are drawbacks of endovascular repair.
Minimal aortic injury can present a management dilemma as there is little data on the natural history of these injuries. However, a few small series suggest that medical management with imaging followup is a safe and appropriate (15) option for management of minimal aortic injuries. Endovascular repair is another option for treatment of minimal aortic injury.
Take home points
- TAIs have a high mortality rate and a high index of suspicion and careful evaluation is needed for accurate diagnosis.
- Direct findings of aortic injury include intramural hematoma, intimal flap and pseudoaneurysm.
- Indirect TAI findings are periaortic hematoma, change in aortic caliber and irregular aortic contour.
- TAIs can be mimicked by ductal diverticula, arterial infundibuli, and venous mediastinal hematoma.
- Technical factors such as the use of ECG gating and obtaining reconstructions with different slice thickness or in a different plane may be helpful.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Parmley LF, Mattingly TW, Manion WC, et al. Nonpenetrating traumatic injury of the aorta. Circulation 1958;17:1086-101. [PubMed]
- Cleverley JR, Barrie JR, Raymond GS, et al. Direct findings of aortic injury on contrast-enhanced CT in surgically proven traumatic aortic injury: a multi-centre review. Clin Radiol 2002;57:281-6. [PubMed]
- Feczko JD, Lynch L, Pless JE, et al. An autopsy case review of 142 nonpenetrating (blunt) injuries of the aorta. J Trauma 1992;33:846-9. [PubMed]
- Crass JR, Cohen AM, Motta AO, et al. A proposed new mechanism of traumatic aortic rupture: the osseous pinch. Radiology 1990;176:645-9. [PubMed]
- Cohen AM, Crass JR, Thomas HA, et al. CT evidence for the “osseous pinch” mechanism of traumatic aortic injury. AJR Am J Roentgenol 1992;159:271-4. [PubMed]
- Creasy JD, Chiles C, Routh WD, et al. Overview of traumatic injury of the thoracic aorta. Radiographics 1997;17:27-45. [PubMed]
- Symbas PN, Tyras DH, Ware RE, et al. Traumatic rupture of the aorta. Ann Surg 1973;178:6-12. [PubMed]
- Burkhart HM, Gomez GA, Jacobson LE, et al. Fatal blunt aortic injuries: a review of 242 autopsy cases. J Trauma 2001;50:113-5. [PubMed]
- Williams JS, Graff JA, Uku JM, et al. Aortic injury in vehicular trauma. Ann Thorac Surg 1994;57:726-30. [PubMed]
- de Mestral C, Dueck AD, Gomez D, et al. Associated injuries, management, and outcomes of blunt abdominal aortic injury. J Vasc Surg 2012;56:656-60. [PubMed]
- Katsumata T, Shinfeld A, Westaby S. Operation for chronic traumatic aortic aneurysm: when and how? Ann Thorac Surg 1998;66:774-8. [PubMed]
- Goodman PC, Jeffrey RB, Minagi H, et al. Angiographic evaluation of the ductus diverticulum. Cardiovasc Intervent Radiol 1982;5:1-4. [PubMed]
- Duvernoy O, Coulden R, Ytterberg C. Aortic motion: a potential pitfall in CT imaging of dissection in the ascending aorta. J Comput Assist Tomogr 1995;19:569-72. [PubMed]
- Demetriades D, Velmahos GC, Scalea TM, et al. Operative repair or endovascular stent graft in blunt traumatic thoracic aortic injuries: results of an American Association for the Surgery of Trauma Multicenter Study. J Trauma 2008;64:561-70; discussion 570-1. [PubMed]
- Paul JS, Neideen T, Tutton S, et al. Minimal aortic injury after blunt trauma: selective nonoperative management is safe. J Trauma 2011;71:1519-23. [PubMed]


