A “low and slow” approach to successful medical treatment of primary cardiac lymphoma
Background
Primary cardiac lymphomas (PCL) are very rare lymphoid malignancies that originate from the endocardium or pericardium (1). The clinical diagnosis is often difficult, as they infrequently present with signs and symptoms consistent with other cardiopulmonary conditions. Typically when B symptoms develop, (fever, weight loss, fatigue common in lymphoid malignancies), progressive heart failure will ensue. Antineoplastic treatments used to treat PCL carry the risk of rapid tumor destruction, causing significant cardiovascular complications (2). We present the case of a 62-year-old male who presented with prolonged fatigue and severe right-sided heart failure as a physiologic consequence of PCL. This report highlights the importance of using multiple diagnostic modalities and presents a conservative, patient-specific treatment approach in the management of such cases.
Case report
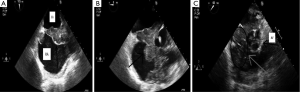
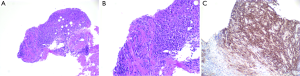
A 62-year-old Caucasian male with coronary artery disease status post bypass grafting presented with a history of several months duration of progressive fatigue, night sweats and unintentional weight loss of twenty pounds. Physical examination was notable for a jugular venous pressure of 20 cmH2O and was otherwise unremarkable for exam findings consistent with heart failure. Laboratory studies were significant for a white blood cell count of 7.2 (range, 4.4-11.3) ×109/L, LDH 560 (range, 84-246) U/L, and HIV, hepatitis B and C serology were negative. A transthoracic echocardiogram (TTE) demonstrated a large right atrial mass adjacent to the tricuspid septal leaflet resulting in severe tricuspid stenosis (mean gradient 15 mmHg) (Figure 1). Positron emission tomography (PET) showed FDG uptake in both the interatrial septum and tricuspid valve (Figure 2). Right heart catheterization with simultaneous transesophageal echocardiography (TEE) further delineated the mass as invading the interatrial septum and encasing the aortic root. As a functional consequence of the mass, hemodynamically significant tricuspid stenosis was measured to be (Fick cardiac index 1.27 L/min/m2). Endomyocardial biopsy demonstrated large cells with prominent eosinophilic nucleoli. Immunohistochemical stains revealed many cells staining positive for CD20 and bcl-2. The EBV in situ hybridization was negative (Figure 3). Flow cytometry of the endomyocardial tissue detected an abnormal, kappa—restricted lymphoid population of cells which were CD5 and CD10 negative and CD19 and CD20 positive (Figure 4). Final histopathological diagnosis was consistent with a diffuse large B cell lymphoma.

The patient was considered ineligible for resection of his cardiac mass, given his comorbidities and the extent of septal involvement. Due to the possibility of ventricular wall rupture secondary to rapid tumor destruction, he was initially monitored in the Cardiac Intensive Care Unit (CICU) and first treated conservatively with rituximab 375 mg/m2 and prednisone 40 mg daily for 10 days, with plans for progressive intensification of chemotherapy if there were no acute complications. The patient tolerated initial treatment without complications, which resulted in the rapid improvement of clinical symptoms.
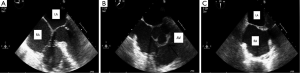
A follow up TTE at 3 weeks demonstrated no change in tumor size however there was no evidence of ventricular septal perforation. According to the proposed therapeutic plan, slow intensification of chemotherapy would be started to avoid potential cardiovascular complications. Two cycles of low dose R-CHOP (cyclophosphamide 400 mg/m2, doxorubicin 25 mg/m2, vincristine 1 mg, prednisone 60 mg and rituximab 375 mg/m2) administered every three weeks were given, and upon good tolerance of this regimen, treatment was escalated to full dose R-CHOP for four cycles given every 3 weeks. Restaging PET at 6 weeks completion of chemotherapy was significant for interval resolution of FDG uptake in the heart (Figure 4). Repeat TEE at 6 months demonstrated resolution of intracardiac lymphoma (Figure 5). Currently, the patient is alive and well without signs of recurrence 12 months after complete remission was indicated by PET.

Summary
PCL is an uncommon intracardiac malignancy and presents with nonspecific signs and symptoms. Timely diagnosis is critical, given that PCL can be rapidly fatal if left untreated. Cardiac rupture, significant arrhythmias, pericardial effusion with tamponade or cardiogenic shock from refractory heart failure remain the predominate reasons for death. Prior to the advent of routine echocardiography, most cases of PCL were reported at autopsy (3). Today, echocardiography, both surface and invasive, have become mainstays of early evaluation even in the context of nonspecific symptomology. Right heart catheterization with biopsy for immunohistochemical staining is readily available for tissue diagnosis (4,5). Response and survival of PCL appears to depend on the underlying patient factors. In particular the presence of immunodeficiency, extracardiac extention, left ventricular (LV) involvement, and arrhythmia impart poor clinical prognosis (4). The presence or absence of LV involvement appeared to have the largest impact in survival. Patients with LV involvement had a median survival of 1 month, whereas those without LV involvement had a median survival of 22 months. Likewise, patients with arrhythmias had a 1 month median survival, whereas those free of arrhythmias had median survival of 6 months.
In the patient presented above, the diagnosis was made during the investigation of clinically severe right-sided heart failure. The treatment approach, although based on a standard treatment regimen for similar B-cell lymphomas, must be appropriately tailored for each patient (6,7). Specifically, our patient was treated with “low-dose and slow increase” approach due to the extent of myocardial infiltration and possiblity of ventricular septal rupture. The patient’s clinical response with the initial chemotherapy regimen was monitored in the CICU and then closely followed. Eventually, the patient was successfully treated with a standard full-dose regimen of chemotherapy and achieved complete remission (7). While chemotherapy is recommended to treat PCL, prognosis and response to treatment are impacted by the cardiovascular complications. Patient monitoring in the CICU while undergoing initial cycles of chemotherapy may offer benefit for early diagnosis and treatment of life threatening arrhythmias, pericardial effusion or ventricular septal rupture.
Acknowledgements
Authors’ contributions: Contribution to the conception and design: Khanjan Shah, Kamal Shemisa; drafting the article or revising it critically for important intellectual content: Khanjan Shah, Kamal Shemisa; final approval of the version to be published: Khanjan Shah, Kamal Shemisa.
Disclosure: The authors declare no conflict of interest.
References
- Ban-Hoefen M, Bernstein SH, Bisognano JD, et al. Symptomatic intracardiac diffuse large B-cell lymphoma. Am J Hematol 2009;84:683-5. [PubMed]
- Butany J, Nair V, Naseemuddin A, et al. Cardiac tumours: diagnosis and management. Lancet Oncol 2005;6:219-28. [PubMed]
- Miguel CE, Bestetti RB. Primary cardiac lymphoma. Int J Cardiol 2011;149:358-63. [PubMed]
- Petrich A, Cho SI, Billett H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer 2011;117:581-9. [PubMed]
- Nascimento AF, Winters GL, Pinkus GS. Primary cardiac lymphoma: clinical, histologic, immunophenotypic, and genotypic features of 5 cases of a rare disorder. Am J Surg Pathol 2007;31:1344-50. [PubMed]
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235-42. [PubMed]
- Nonami A, Takenaka K, Kamezaki K, et al. Successful treatment of primary cardiac lymphoma by rituximab-CHOP and high-dose chemotherapy with autologous peripheral blood stem cell transplantation. Int J Hematol 2007;85:264-6. [PubMed]


