Electrocardiograhic findings resulting in inappropriate cardiac catheterization laboratory activation for ST-segment elevation myocardial infarction
Introduction
Timely reperfusion is of prime importance in ST-segment elevation myocardial infarction (STEMI) outcomes and hence a door to balloon time (DBT) of <90 min is recommended (1). In an effort to achieve this crucial metric, many cardiac catheterization laboratories (CCL) are now activated by either the emergency medical services (EMS) or the emergency department (ED) for patients with suspected STEMI (2,3). Studies have shown that pre-hospital electrocardiograms (ECG’s) with rapid STEMI activation by EMS or the ED leads to a significant reduction in DBT (4,5). This pattern of care has been proposed by the American Heart Association as part of a system of care for STEMI patients (6). Although this has led to a reduction in time to reperfusion, it may lead to activation of the CCL for clinical scenarios not consistent with STEMI (7). The American Heart Association Mission Lifeline has recommended the terms “appropriate” (APA-CCL) versus “inappropriate” (IPA-CCL) for the CCL activations (8). APA-CCL is based on the ECG findings of STEMI as well as the appropriate clinical findings for this diagnosis. However, there has not been a standardized definition for IPA-CCL used in the literature (9).
The diagnosis of STEMI is a syndrome involving clinical symptoms, electrocardiographic changes and occlusion of a culprit artery. The vagaries of the clinical symptoms may complicate the early diagnosis particularly in women, the elderly and diabetic patients (10). Likewise, the ECG can also be misleading as there are many imitators of STEMI (11,12).
Despite defined criteria, there continues to be misinterpretation of the ECG resulting in either a failure to activate in the setting of a STEMI or in IPA-CCL. In addition the initial ECG may not be diagnostic which further complicates making an early diagnosis (13). There are a paucity of studies investigating the ECG findings that result in IPA-CCL. There are even fewer studies that examine both the clinical symptomatology and ECG findings leading to IPA-CCL.
Several studies have examined the rates of IPA-CCL and various descriptions of what constitutes an IPA-CCL have been used (7,14-17). In this study, we describe the clinical and ECG findings that led to CCL activation in the first 18 months after the inception of our primary percutaneous coronary intervention (PCI) program.
Materials and methods
Study design
The data presented are from a tertiary care safety net hospital that has been performing elective PCIs since September 2007 and initiated a primary PCI program in January 2011. We performed a prospective observational analysis of all the STEMI activations for the first 18 months of the program. A data base of all STEMI activations was made which also included the clinical information as well as the ECG and angiographic findings when a catheterization was done. The data base was used as part of a quality assurance program for the cardiology section to assess CCL activation.
All the STEMI activations were made by either EMS or the ED. In the activations done in the ED the attending ED staff initiated the activation. There was a single call made to the hospital operator who then activated the pagers of the STEMI team. All ECGs were reviewed by a cardiology fellow and staff when they arrived at the hospital either in the ED or as the patient was being transferred to the CCL. Clinical symptoms at presentation were obtained by interview of the patients by the on-call cardiology fellow and the attending interventional cardiology staff member prior to the planned catheterization procedure. All the activations in this report were within the Truman Medical Center system which has two campuses, 17 miles apart. Twenty three of the total activations involved ambulance transfer to the CCL located at the larger campus.
Serial troponin I measurements were done using the Siemens Ultra I instrument on all patients with the upper limits of normal being 0.06 µg/L with the lower limits of detection being 0.006 µg/L and the 99th percentile reference value of 0.040 µg/L (18).
Definitions
APA-CCL was defined as all activations with clinical and ECG findings on presentation as well as the results of the cardiac catheterization showing a culprit lesion in the same distribution as the ECG changes consistent with a STEMI diagnosis. No core laboratory was utilized for the ECG or angiogram interpretation. There was internal agreement in the ECG interpretation between the interventional cardiologists on call and the authors (SS, JM and DB).
IPA-CCL was defined as all activations which were not consistent with a STEMI diagnosis based on either the angiographic results or the clinical/ECG findings at presentation. All patients had serial troponin testing and were followed by the cardiology staff to assure that the appropriate clinical diagnosis had been made during the hospitalization. The patient’s data was also reviewed prior to hospital discharge to make certain there were no changes in the activation classification.
Atypical symptoms in the patients deemed to have IPA-CCL included any symptom but typical chest pain, dyspnea, ventricular tachycardia or cardiac arrest (the later four were considered as potential STEMI symptoms).
In those patients who underwent coronary angiography, mild coronary artery disease (CAD) was defined as no stenosis of greater than 50% diameter stenosis.
Coronary spasm was defined as a reversible stenosis responsive to intracoronary nitroglycerin proven by cardiac catheterization either in the acute hospitalization or on a prior hospitalization.
After hours CCL activation was defined as occurring between 6 PM and 7 AM or on the weekend or hospital holidays.
ECG review criteria
The ECG diagnosis of STEMI was based on the Second Universal Definition of Myocardial Infarction (10). The criteria for left bundle branch block (LBBB) and LBBB with changes of infarction were made as described by Sgarbossa et al. (19). The criteria for left ventricular hypertrophy (LVH) utilized the Cornell and Sokolow-Lyon criteria (20,21).
Statistical analysis
The Chi Square test was used and two-tailed analysis was performed. We created 2×2 table and analyzed the data using the freely available online tool on graphpad.com (http://graphpad.com/quickcalcs/contingency1.cfm).
Results
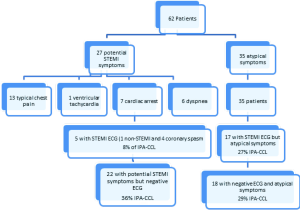
There were a total of 139 STEMI activations over the 18 months period of the study. Seventy seven (55%) were classified as APA-CCL. Sixty two (45%) were deemed IPA-CCL as no STEMI diagnosis was confirmed during the hospitalization.
APA-CCL: 77 patients (55% of the total activations)
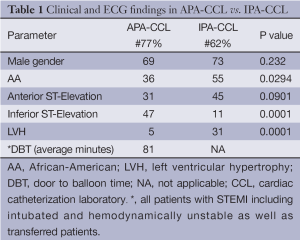
The mean age in this group was 54.3 years and 69% were males. There were 36% African-Americans (AA), 52% Caucasian and 12% Hispanic. There were significantly more Caucasians in the APA-CCL as compared to those in the IPA-CCL cohort (P=0.0198) and more AA in the IPA-CCL group than with APA-CCL (P=0.0294). The DBT for this cohort was 81 minutes as shown in Table 1. This was for all APL-CCL patients including those with hemodynamic instability, emergently intubated as well as those transferred.
Full table
IPA-CCL: 62 patients (45% of the total activations)
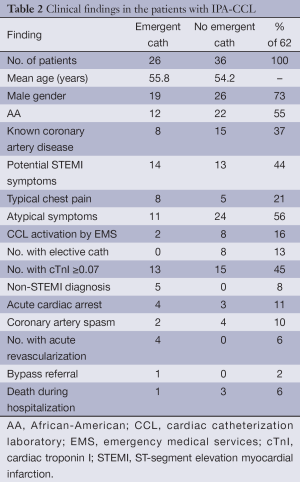
There were two groups of these patients: one group (36 patients) did not undergo acute catheterization as the CCL activation was cancelled before the catheterization. The second group did undergo emergent catheterization (26 patients). The clinical findings of both groups are shown in Table 2 and are similar. Over half of the patients with IPA-CCL had atypical symptoms (56%) and 21% of the group had typical chest pain. Patients with potential STEMI symptoms did account for 44% of the IPA-CCL (Table 2). There was no correlation in the incidence of IPA-CCL with the starting dates of the residents and fellows. Of the IPA-CCL activations 52 were made in the ED and 10 by EMS (Table 2).
Full table
We did not observe any patients who had a final STEMI diagnosis where there was a failure to activate the CCL on presentation.
Emergent catheterization group (42% of the IPA-CCL): (Table 2)
This group included five patients with non-STEMI’s of whom four had an emergent PCI. The other non-STEMI was stabilized and then referred for coronary artery bypass surgery (CABG). Four patients presented with an out of hospital cardiac arrest and none of these had significant CAD. Two patients had coronary spasm. The remaining 15 patients in this group who underwent an emergent catheterization had either no angiographic CAD, stable disease from a prior catheterization or only mild CAD (<50% diameter stenosis). Eight of the 26 were known to have CAD.
No emergent catheterization group (58% of the IPA-CCL (Table 2)
The activation was cancelled by the interventional cardiologist either because the because of an atypical presentation that did not favor a STEMI diagnosis and/or the ECG did not meet STEMI criteria. None of these patients had a STEMI during the hospitalization. Fifteen of these patients were known to have CAD. Three patients had experienced an acute cardiac arrest (none with a STEMI ECG) and four had previously documented coronary artery spasm.
In this group 8 ultimately underwent cardiac catheterization during the index hospitalization and none of these patients had revascularization. The findings of the catheterizations showed either no angiographic CAD, no change from a recent angiogram or mild CAD.
The 62 patients with IPA-CCL in this study were a critically ill group of patients and nearly 50% of the entire group had elevated troponin levels. The major causes for the troponin elevation besides the non-STEMI group were demand ischemia (including hypertension and hypotension), heart failure, coronary artery spasm, and respiratory failure. Four of 62 died during the hospitalization (one with pulmonary embolism and three due to the out of hospital cardiac arrest.
ECG analysis
An analysis of the ECG findings in the APA-CCL versus IPA-CCL is shown in Table 1. In the APA-CCL inferior STEMI was the most common at 47% (36 of 77), while 31% had anterior STEMI. The remainder of the STEMI patients had either anterior-lateral, inferior-lateral or inferior-posterior involvement. LVH was significantly more common in the IPA-CCL group with 19 patients having LVH as compared with 4 in the APA-CCL group (P=0.0001).
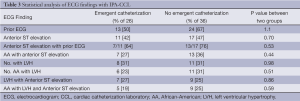
The distribution of ECG findings with both groups of IPA-CCL is seen in Table 3. Prior ECG’s were present in 60% of the group at the time of activation. Anterior ST-segment elevation was the most common ECG finding in both groups. Twenty of the 28 patients with anterior ST-elevation had prior ECG’s. Of the patients with anterior ST-segment elevation 20 were AA. Nineteen (31%) had ECG criteria for LVH and 17 of these were AA. Of the LVH patients 16 of the 19 had anterior ST-Segment elevation 30.2 mV and 14 of these were AA. Table 3 also shows that there was no statistical difference in the ECG findings between the two groups with IPA-CCL.
Full table
Table 4 shows additional ECG results in the IPA-CCL. Anterior ST-segment elevation and the combination of LVH plus anterior ST-elevation contributed to false activations. Both of these findings were more common in AA. There were 22 patients out of the 62 IPA-CCL whose ECG fulfilled STEMI criteria and there was a strong association of these criteria with LVH and with the AA race.
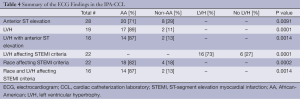
Full table
Of the 22 patients with a STEMI-ECG and IPA-CCL 21 had anterior ST-segment elevation. Of the five patients who had a non-STEMI, only one had a STEMI-ECG. This patient had LVH and anterior ST-segment elevation. All of his coronary arteries were patent at the time of the cardiac catheterization but because of severe three vessel CAD he was stabilized and then referred for coronary bypass.
Four patients with coronary spasm had dynamic ST-segment elevation that met the STEMI criteria. None of these patients had LVH. Three had anterior ST-segment elevation and one had inferior ST-segment elevation. The two who underwent emergent catheterization were first time presentations while the two who did not undergo emergent catheterization were known to have coronary artery spasm.
Of these 22 patients with an ECG that fulfilled the STEMI criteria, 77% had atypical symptoms. The five patients with typical symptoms had either coronary spasm or a non-STEMI. Figure 1 is a flow diagram showing the clinical symptoms in the IPA-CCL cohort as they related to the STEMI-ECG findings.
As shown in Table 5 in the total activation cohort of 139 patients there were 99 patients (71%) whose ECG fulfilled STEMI criteria and 40 (29%) who did not meet these criteria. There were 104 patients with symptoms of a potential STEMI (77 in the APA-CCL group and 27 in the IPA-CCL group). This was 75% of the total activation cohort. There were 103 patients (74%) of all activations who actually had an emergent catheterization during the study period.
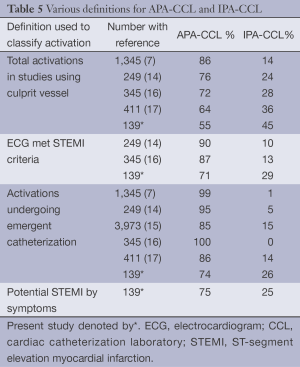
Full table
Discussion
In an effort to make a rapid diagnosis of a STEMI and to meet the 90 minutes DBT, IPA-CCL may result. We report a high incidence of IPA-CCL in the first 18 months after the initiation of a STEMI program at our institution. IPA-CCL was defined as either a lack of a culprit vessel if the patient underwent emergent catheterization or the lack of clinical findings (ECG and/or clinical presentation) if no catheterization was performed emergently. The most common electrocardiographic reason for the IPA-CCL was anterior ST-segment elevation. This was a particular problem in AA with LVH. In addition, 56% of the patients with IPA-CCL presented with atypical symptoms. There has been a paucity of data on the ECG findings leading to IPA-CCL and the results here underscore the diagnostic conundrum that is presented by anterior ST-segment elevation.
The definitions used in the literature for IPA-CCL have been variable but most commonly have used the lack of a culprit artery at the time of catheterization to indicate an IPA-CCL. Table 5 summarizes the studies that have evaluated IPA-CCL (7,14-17). Four different definitions are used to delineate IPA-CCL. These include: lack of a culprit vessel, ECG not fulfilling STEMI criteria, activations where no emergent catheterization is performed and lack of symptoms consistent with a STEMI diagnosis. The variance in the percentages of IPA-CCL based on the definition used is illustrated in Table 5.
Mixon et al. proposed a two-step refinement in the approach to IPA-CCL: the first is based on ECG appropriateness and the second on the final diagnosis based on the emergent angiography (16).
McCabe et al. defined the false positives as failure to find a culprit vessel in those patients who underwent angiography or by clinical variables in the patients without angiograms (17). They did examine the ECG as well as the clinical findings that lead to an IPA-CCL. The presence of left ventricular hypertrophy was a common ECG finding in the IPA-CCL patients. In addition. anterior ST-segment elevation was seen in 60% of their false activations. These authors found that 12% of the patients with APA-CCL were AA and in contrast 27% of the IPA-CCL were AA.
The problem of ST-segment elevation in patients with LVH was evaluated by Armstrong et al. (22) using the ACTIVATE-SF data base used in the study of McCabe et al. (17). They proposed ECG criteria using a ratio of the ST-segment elevation to the RS-magnitude in the lead with the greatest ST-segment elevation. If the ratio was ≥25% they felt this would support a diagnosis of STEMI in the presence of LVH.
In our report on IPA-CCL activations the most common ECG cause for these activations was ST-segment elevation in the right precordium and this was seen in 45% similar to the data of McCabe et al. (17). Left ventricular hypertrophy occurred in 31% of the false activations and was also frequently seen in the McCabe report. LVH is a known cause of anterior ST-segment elevation. We observed that anterior ST-segment elevation was commonly seen in AA particularly with LVH and commonly resulted in IPA-CCL. These observations are consistent to those of McCabe et al. (17). We applied the findings of Armstrong et al. (22) to the 16 patients in our study with LVH and anterior ST-segment elevation and found that the ratio was <25% in all of them.
The clinical findings which produced the high rate of IPA-CCL in this study are illustrated in Figure 1. Analysis of this data shows that only five patients had both potential STEMI symptoms and a STEMI ECG (8% of IPA-CCL) while 18 had neither symptoms nor ECG suggestive of STEMI (29% of IPA-CCL). The remaining approximately 60% had either a STEMI ECG and atypical symptoms and or concerning symptoms and a negative ECG. This data underscores the difficulty in the rapid evaluation and triage of a patient who may be presenting with a STEMI. These patients are a heterogeneous and complicated group of patients. They are also quite ill as illustrated by nearly 50% of the IPA-CCL having elevated troponins. This is compounded by the urgency of rapid reperfusion and in this case making sure that a DBT of <90 minutes is achieved. The clinician has only the clinical history/examination and the ECG on which to base the decision for CCL activation. In this study there were problems both with the interpretation of the history as well as the ECG and both contributed to the IPA-CCL.
With respect to the history, this was a new program and the clinicians were not experienced in initiating a STEMI activation in an expeditious fashion. The attendant learning curve for the fellows and residents may have further contributed. Garvey et al. (15) noted that IPA-CCL was more likely at a non-primary PCI hospital and our data suggests this would apply to a new primary PCI program.
The patient population in this study likely contributed to the ECG misinterpretation. As mentioned, this study had a high rate of AA patients with LVH and this complicated the ECG interpretation. Perhaps the new criteria for leads V2-V3 of ≥0.25 mV in men <40 years will help in the interpretation of ST-elevation in the anterior leads (12). With educational programs emphasizing the association of LVH and anterior ST-segment elevation, and by applying the criteria proposed by Armstrong et al. (22) the number of IPA-CCL likely would be reduced. Sixty percent of the patients with IPA-CCL had prior ECGs. Taking time to carefully compare the old ECGs would likely improve the ECG interpretation as well. Electronic transmission of the ECG to the smart phone of the interventional cardiologist may also improve the accuracy of the ECG interpretation.
The IPA-CCL comes at a cost most importantly for the patient who is potentially subjected to an unneeded procedure. Emergent procedures are known to increase the patient risk. A recent meta-analysis showed poorer outcomes in patients presenting during off-hours with an acute myocardial infarction (23). In our series 69% of these activations were after-hours. It has also been shown that patients presenting after-hours were more likely to be minority groups and have an increased comorbidities (24). Our high percentage of after-hours activations may have also contributed to the increased number of IPA-CCL.
There is also an added burden for the personnel and the institution these activations. The catheterization laboratory cost of an after-hours IPA-CCL activation was approximately $350 if no catheterization was done and about $865 if one was performed.
The STEMI process likely would be improved by taking some extra time in the initial evaluation to make certain that the clinical findings and ECG fit a potential STEMI diagnosis. As John Wooden, the famous UCLA basketball coach said “be quick but don’t hurry”.
It is apparent from the literature review and from our data, that there is no perfect classification system for IPA-CCL. We believe that each program should evaluate their IPA-CCL results by adding a third tier (clinical symptoms) to that proposed by Mixon et al. (16). Ideally, the rate of IPA-CCL would be nearly the same for all three tiers and should approach 20% in an experienced center.
Limitation of this study
This is a single center study with a limited number of patients studied. In addition, the initiation of a new STEMI program in a teaching institution with the attendant learning curve for all the physicians and staff involved in such an undertaking may have contributed to the rate of IPA-CCL. However, the data of McCabe in a more established center (17), was only slightly better in terms of IPA-CCL than that reported here.
Conclusions
A high incidence of IPA-CCL was seen in this report of STEMI activations in the first 18 months after the commencement of a primary PCI program. A combination of atypical symptoms and ECG misinterpretation contributed. The most common electrocardiographic reason for IPA-CCL was anterior ST-segment elevation accounting for 45% of these activations. There was a strong association of this finding with LVH in the AA population. Of the 35% of patients with IPA-CCL whose ECG which met the STEMI criteria, 95% had anterior ST-segment elevation. Here again LVH in the AA population contributed to this finding. The majority of these patients had an atypical presentation which made a STEMI diagnosis unlikely. The factors potentially contributing to the IPA-CCL rate were: initial experience in a new program, frequency of after-hours activations, high percentage of AA in the population and under appreciation of the contribution of LVH to anterior ST-segment elevation.
Acknowledgements
To Drs. Javed Ashraf and Paramdeep Baweja for their excellent interventional care.
Disclosure: The authors declare no conflict of interest.
References
- Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009;54:2205-41. [PubMed]
- Krumholz HM, Bradley EH, Nallamothu BK, et al. A campaign to improve the timeliness of primary percutaneous coronary intervention: Door-to-Balloon: An Alliance for Quality. JACC Cardiovasc Interv 2008;1:97-104. [PubMed]
- Holmes DR Jr, Bell MR, Gersh BJ, et al. Systems of care to improve timeliness of reperfusion therapy for ST-segment elevation myocardial infarction during off hours: the Mayo Clinic STEMI protocol. JACC Cardiovasc Interv 2008;1:88-96. [PubMed]
- Hutchison AW, Malaiapan Y, Jarvie I, et al. Prehospital 12-lead ECG to triage ST-elevation myocardial infarction and emergency department activation of the infarct team significantly improves door-to-balloon times: ambulance Victoria and MonashHEART Acute Myocardial Infarction (MonAMI) 12-lead ECG project. Circ Cardiovasc Interv 2009;2:528-34. [PubMed]
- Pitta SR, Myers LA, Bjerke CM, et al. Using prehospital electrocardiograms to improve door-to-balloon time for transferred patients with ST-elevation myocardial infarction: a case of extreme performance. Circ Cardiovasc Qual Outcomes 2010;3:93-7. [PubMed]
- Jacobs AK, Antman EM, Faxon DP, et al. Development of systems of care for ST-elevation myocardial infarction patients: executive summary. Circulation 2007;116:217-30. [PubMed]
- Larson DM, Menssen KM, Sharkey SW, et al. “False-positive” cardiac catheterization laboratory activation among patients with suspected ST-segment elevation myocardial infarction. JAMA 2007;298:2754-60. [PubMed]
- American Heart Association Mission:Lifeline. Inappropriate Activation Form. Available online: www.heart.org/idc/groups/heart-public/@wcm/@global/documents/downloadable/ucm_316080pdf
- Bachour F, Asinger R. Activating primary percutaneous coronary intervention for STEMI that is not: the collateral damage of improving door-to-balloon time: comment on “Prevalence and factors associated with false-positive ST-segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention-capable centers”. Arch Intern Med 2012;172:871-2. [PubMed]
- Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation 2007;116:2634-53. [PubMed]
- Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med 2003;349:2128-35. [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581-98. [PubMed]
- Riley RF, Newby LK, Don CW, et al. Diagnostic time course, treatment, and in-hospital outcomes for patients with ST-segment elevation myocardial infarction presenting with nondiagnostic initial electrocardiogram: a report from the American Heart Association Mission: Lifeline program. Am Heart J 2013;165:50-6. [PubMed]
- Kontos MC, Kurz MC, Roberts CS, et al. An evaluation of the accuracy of emergency physician activation of the cardiac catheterization laboratory for patients with suspected ST-segment elevation myocardial infarction. Ann Emerg Med 2010;55:423-30. [PubMed]
- Garvey JL, Monk L, Granger CB, et al. Rates of cardiac catheterization cancelation for ST-segment elevation myocardial infarction after activation by emergency medical services or emergency physicians: results from the North Carolina Catheterization Laboratory Activation Registry. Circulation 2012;125:308-13. [PubMed]
- Mixon TA, Suhr E, Caldwell G, et al. Retrospective description and analysis of consecutive catheterization laboratory ST-segment elevation myocardial infarction activations with proposal, rationale, and use of a new classification scheme. Circ Cardiovasc Qual Outcomes 2012;5:62-9. [PubMed]
- McCabe JM, Armstrong EJ, Kulkarni A, et al. Prevalence and factors associated with false-positive ST-segment elevation myocardial infarction diagnoses at primary percutaneous coronary intervention–capable centers: a report from the Activate-SF registry. Arch Intern Med 2012;172:864-71. [PubMed]
- Apple FS, Smith SW, Pearce LA, et al. Use of the Centaur TnI-Ultra assay for detection of myocardial infarction and adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem 2008;54:723-8. [PubMed]
- Sgarbossa EB, Pinski SL, Barbagelata A, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med 1996;334:481-7. [PubMed]
- Casale PN, Devereux RB, Kligfield P, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol 1985;6:572-80. [PubMed]
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949;37:161-86. [PubMed]
- Armstrong EJ, Kulkarni AR, Bhave PD, et al. Electrocardiographic criteria for ST-elevation myocardial infarction in patients with left ventricular hypertrophy. Am J Cardiol 2012;110:977-83. [PubMed]
- Sorita A, Ahmed A, Starr SR, et al. Off-hour presentation and outcomes in patients with acute myocardial infarction: systematic review and meta-analysis. BMJ 2014;348:f7393. [PubMed]
- Jneid H, Fonarow GC, Cannon CP, et al. Impact of time of presentation on the care and outcomes of acute myocardial infarction. Circulation 2008;117:2502-9. [PubMed]