
Prenatal duct closure leading to severe pulmonary hypertension in a preterm neonate—a case report
Introduction
The ductus arteriosus (DA) plays a vital role in normal fetal circulation by allowing the majority of fetal right ventricular (RV) output to bypass the lungs. DA patency is maintained in utero by low oxygen tension of the blood, endothelial NO production, and prostaglandins E1 and E2. Interruption of prostaglandin synthesis during pregnancy, whether from non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or polyphenol-rich foods, can result in premature closure of the DA. In utero, DA closure leads to fetal lung over-circulation, which increases muscularization of the pulmonary vasculature and predisposes the infant to persistent pulmonary hypertension (PH) of the newborn (1). Prolonged exposure to DA closure in utero leads to RV hypertrophy and dysfunction, and in severe cases fetal hydrops, fetal demise, or death in the immediate postnatal period. Although the clinical presentation and severity of in utero DA closure is variable, mortality has been reported as high as 10–25% (1-3). Due to the rarity of this condition, there is no widely accepted standard treatment for infants who develop the adverse cardiovascular sequelae of prenatal DA closure after birth. Furthermore, the majority of reported cases describe therapeutic measures for infants born full term, whereas reports of successful therapeutic strategies among premature infants are lacking. We present the following case in accordance with the CARE Guideline (available at http://dx.doi.org/10.21037/cdt-20-123).
Case presentation
A female diamniotic-dichorionic twin was delivered by Cesarean section at 31 weeks 5 days gestational age (GA) to a 32-year-old gravida 9 para 8 mother. Both parents are of African American descent and were not consanguineous. Pregnancy was complicated by maternal malnutrition secondary to an unspecified eating disorder, tobacco exposure (1.5 packs per day), intrauterine growth restriction of both twins, and a non-reassuring fetal heart tracing which, in combination with absent end diastolic flow in the umbilical artery, ultimately prompted preterm delivery. Routine prenatal screening tests were unremarkable. Chorionic villus sampling at 13 weeks demonstrated the infant’s karyotype to be 46XX. Fetal anatomic survey at 18 weeks was unremarkable, including normal cardiac structures. Maternal medications during pregnancy included citalopram (for depression), aspirin (prescribed due to history of preeclampsia), and over-the-counter acetaminophen and ibuprofen (exact amounts unknown). Mother received betamethasone at 28 weeks with an incomplete second course before delivery. She did not receive indomethacin for tocolysis.
The infant’s Apgar scores at 1 and 5 minutes were 5 and 7. The infant’s birth weight was 1,190 grams (20th percentile), length 38 cm (24th percentile) and head circumference 26.5 cm (17th percentile) (4). The infant had no physical exam findings to suggest an underlying genetic condition. She required intubation for respiratory failure and received two doses of surfactant. Concern for congenital heart disease arose within the first 12 hours of life as the infant developed worsening hypoxemia with oxygen saturations in the mid-80’s and arterial partial pressure of oxygen (PaO2) of 50 mmHg on 100% fraction of inspired oxygen (FiO2). Pre- and post-ductal saturations showed no differential. Electrocardiogram revealed sinus tachycardia. Chest X-ray revealed clear lungs and mild cardiomegaly. Transthoracic echocardiogram obtained at 15 hours of life revealed absence of the DA, an abnormal RV with severe hypertrophy, diastolic dysfunction and mild systolic dysfunction, a patent foramen ovale with right-to-left flow, and mild tricuspid regurgitation. The estimated RV pressure by tricuspid regurgitation jet was at least 35 mmHg plus the right atrial v-wave, likely an underestimate as the Doppler signal was somewhat weak; other evidence of systemic RV pressure included flat septal position in systole and a suspicion of a tiny muscular ventricular septal defect with flow from the RV to the left ventricle (LV), suggesting systemic-level RV pressure on the initial study. The pulmonary valve leaflets were thin but did not open completely, likely a functional issue related to reduced RV outflow as there was never a significant gradient across the valve and repeat echocardiogram 10 days later showed normal valvar function without gradient. There was normal LV size and systolic function, which is standardly measured according to the American Society of Echocardiography standards by LV volumes and ejection fraction (EF) using the 5/6 area × length method (LV EF 58% on the initial study). Diastolic indices were not performed on the initial urgent study, and transmitral Doppler E/A ratio demonstrated fusion of the E/A waves which is a frequent finding in this neonatal age group particularly in the setting of sinus tachycardia. Thus, interpretation of diastolic indices was not possible (Figure 1).
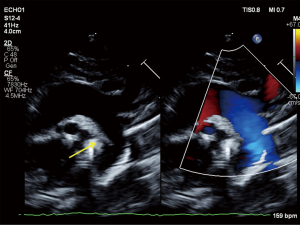
Due to persistent hypoxemia and RV dysfunction at 24 hours of life, a milrinone infusion was started at 0.33 mcg/kg/min IV without a loading dose. The infant did not experience systemic hypotension during treatment. The infant’s hypoxemia subsequently improved and milrinone was discontinued after 3 days. Echocardiogram on day of life (DOL) 11 showed improvement with normal biventricular function and no evidence of elevated RV pressure; repeat echocardiogram on DOL 22 remained stable. On these repeat echocardiograms, there was insufficient tricuspid regurgitation to estimate RV pressure, no significant systolic septal flattening, and no residual VSD flow. The infant was extubated on DOL 8 to continuous positive airway pressure (CPAP) and subsequently weaned to room air by DOL 13, with an otherwise uneventful recovery. Routine serial head ultrasounds on DOL 1, 4, 8, and 29 were all negative for intraventricular hemorrhage.
The infant was discharged from the neonatal intensive care unit (NICU) at 38 weeks post-menstrual age (PMA), requiring no respiratory support and taking full volume oral feeds. The infant’s twin had no clinical evidence of premature closure of the DA. Placental pathology of the affected twin revealed extensive regional villous sclerosis while the placenta of the unaffected twin revealed only multiple thin-walled simple chorionic cysts and a small recent microscopic placental infarct.
Discussion
Prenatal DA closure is a rare condition and published data regarding the clinical presentation and management are limited to case reports and case series. The majority of published cases describe full term infants, and reports of this condition in the preterm population are extremely limited. Prenatal DA closure is likely even rarer among preterm infants than among term infants because the ductal tissue is less sensitive to factors promoting constriction at earlier GAs. Sustained ductus response to NSAIDs is uncommon before 27 weeks GA (5,6) and remains limited prior to 31 weeks GA (7). Studies have shown that from 24–32 weeks gestation, fetal exposure to NSAIDs often results in reversible ductal constriction without complete closure, which resolves after the exposure is removed (1,5,6). Thus, the incidence of in utero DA closure likely increases with GA. In the rare event that DA closure occurs at <32 weeks GA, the diagnosis is generally made prenatally via ultrasound; subsequently, the majority of infants are managed conservatively in utero and deliver at or close to full term (3), although in some cases progressive cardiac dysfunction prompts preterm delivery (1,2).
The case we present is unique because the DA closure likely occurred early in gestation, and because the diagnosis was made after delivery based on the postnatal clinical presentation. In utero ductus closure leads to RV overload, causing elevated RV pressure, tricuspid regurgitation, and subsequent RV dysfunction. PH results from medial thickening of the pulmonary vessels from increased flow to the premature lungs (8). The severity of RV hypertrophy and PH present on the day of birth suggest the ductal closure occurred weeks prior to delivery. We obtained the infant’s first echocardiogram showing DA closure at only 15 hours of life, suggesting that the duct closed before delivery. Furthermore, the patient’s severe RV hypertrophy suggested the RV had been contracting against high pulmonary pressure in utero for days to weeks, and the PH suggests increased pulmonary blood flow also existed in utero. The diagnosis of prenatal DA closure is supported by the history of multiple exposures known to cause DA closure, including tobacco, ibuprofen, and potential contributions from acetaminophen, aspirin and betamethasone, as well as placental insufficiency. Our case was also interesting in the fact that only one twin developed prenatal closure of the DA. We theorize this is likely related to difference in degree of placental insufficiency and thus hypoxia between twins based upon placental pathology as mentioned above. Timing of fetal DA closure could potentially impact disease severity and survival; earlier closure may increase severity of PH after birth or carry higher risk for fetal demise/hydrops fetalis. However, the authors speculate that further increased intensity of prenatal care would have been unlikely to alter the course of this infant’s care. Due to the twin pregnancy and finding of diminished end-diastolic flow at 26 weeks the pregnancy was monitored closely, at least weekly after that point. A fetal survey at 18 weeks did not identify anomalies. In retrospect, given the number of risk factors in this pregnancy, a fetal echocardiogram could have been considered. Certainly, if the diagnosis were known, follow-up fetal echocardiograms could have been done to determine optimal timing of delivery, but because the twins were already born premature, and the affected twin had no signs of hydrops or systemic impact, it is difficult to say with confidence if delivery would have been indicated prior to 31 weeks.
Infants born after in utero DA closure who are symptomatic have traditionally been treated with inhaled nitric oxide (iNO) (9). iNO is approved in the United States for treatment of PH in more mature infants >34 weeks GA. While a body of evidence suggests iNO is safe among younger GA infants (10), evidence from a few studies suggest an association of iNO use with intraventricular hemorrhage (11-13). The US-based Pediatric Pulmonary Hypertension Network recommend iNO for some subgroups of premature infants with PH associated with oligohydramnios, pulmonary hypoplasia, or sepsis (10). The European Pediatric Pulmonary Vascular Disease Network (EPPVDN) recommends milrinone as an alternative to iNO if there is evidence of impaired systolic ventricular function with PH (14).
In this case, providers chose to use milrinone as a targeted therapy for the infant’s RV hypertrophy and dysfunction. Milrinone is a phosphodiesterase (PDE) 3 inhibitor (with some effect on PDE5 and PDE1 as well) that acts upon both cardiomyocytes and pulmonary arterioles to increase cardiac contractility and diastolic function in addition to pulmonary vasodilation (15). The onset of action is slower than iNO and attention to renal function and clearance are necessary particularly in preterm infants (15,16). Milrinone use for PH has occasionally been reported previously in full term infants (17), and in one case of a preterm infant who was unresponsive to iNO (18). The most common adverse effect of milrinone is systemic hypotension requiring vasopressor medications, though this affects a minority of patients (16). Other adverse effects include hypokalemia, renal dysfunction and thrombocytopenia, none of which occurred in this patient. Our institution (19) and others (16) have had prior success using milrinone in very preterm infants (≤33 weeks PMA) with PH from a variety of etiologies, including pulmonary hypoplasia, recipient of twin-to-twin transfusion, and severe growth restriction due to placental insufficiency. These reports generally show improvement in cardiac function, cardiac output, and oxygen requirement within 2–5 days of milrinone therapy, similar to what we observed in our patient. Nevertheless, studies of milrinone use in larger cohorts of preterm infants would be necessary to fully elucidate safety and efficacy in this population.
Acknowledgments
The authors would like to acknowledge Dr. Helen Christou for the contribution of her medical expertise to the patient’s case and Dr. Terrie Inder, Chair of the Department of Pediatric Newborn Medicine at Brigham and Women’s Hospital.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Martin Koestenberger, Harm-Jan Bogaard and Georg Hansmann) for the series “Right Ventricular Dysfunction” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Editor-in-Chief and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure forms (available at http://dx.doi.org/10.21037/cdt-20-123). The series “Right Ventricular Dysfunction” was commissioned by the editorial office without any funding or sponsorship. MM reports personal fees and other from Actelion, outside the submitted work. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Institutional Review Board approval was not required as the information is reported in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ishida H, Kawazu Y, Kayatani F, et al. Prognostic factors of premature closure of the ductus arteriosus in utero: a systematic literature review. Cardiol Young 2017;27:634-8. [Crossref] [PubMed]
- Genovese F, Marilli I, Benintende G, et al. Diagnosis and management of fetal ductus arteriosus constriction-closure. J Neonatal Perinatal Med 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Gewillig M, Brown SC, De Catte L, et al. Premature foetal closure of the arterial duct: clinical presentations and outcome. Eur Heart J 2009;30:1530-6. [Crossref] [PubMed]
- Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013;13:59. [Crossref] [PubMed]
- Lopes LM, Carrilho MC, Francisco RP, et al. Fetal ductus arteriosus constriction and closure: analysis of the causes and perinatal outcome related to 45 consecutive cases. J Matern Fetal Neonatal Med 2016;29:638-45. [Crossref] [PubMed]
- Luchese S, Mânica JL, Zielinsky P. Intrauterine ductus arteriosus constriction: analysis of a historic cohort of 20 cases. Arq Bras Cardiol 2003;81:405-10, 399-404.
- Vermillion ST, Scardo JA, Lashus AG, et al. The effect of indomethacin tocolysis on fetal ductus arteriosus constriction with advancing gestational age. Am J Obstet Gynecol 1997;177:256-9; discussion 259-61. [Crossref] [PubMed]
- Ishida H, Inamura N, Kawazu Y, et al. Clinical features of the complete closure of the ductus arteriosus prenatally. Congenit Heart Dis 2011;6:51-6. [Crossref] [PubMed]
- Downing GJ, Thibeault DW. Pulmonary vasculature changes associated with idiopathic closure of the ductus arteriosus and hydrops fetalis. Pediatr Cardiol 1994;15:71-5. [PubMed]
- Kinsella JP, Steinhorn RH, Krishnan US, et al. Recommendations for the use of inhaled nitric oxide therapy in premature newborns with severe pulmonary hypertension. J Pediatr 2016;170:312-4. [Crossref] [PubMed]
- Barrington KJ, Finer N, Pennaforte T. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev 2017;1:CD000509. [Crossref] [PubMed]
- Barrington KJ, Finer NN. Inhaled nitric oxide for preterm infants: a systematic review. Pediatrics 2007;120:1088-99. [Crossref] [PubMed]
- Mercier JC, Hummler H, Durrmeyer X, et al. Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. Lancet 2010;376:346-54. [Crossref] [PubMed]
- Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant 2019;38:879-901. [Crossref] [PubMed]
- Joynt C, Cheung PY. Treating hypotension in preterm neonates with vasoactive medications. Front Pediatr 2018;6:86. [Crossref] [PubMed]
- Samiee-Zafarghandy S, Raman SR, van den Anker JN, et al. Safety of milrinone use in Neonatal Intensive Care Units. Early Hum Dev 2015;91:31-5. [Crossref] [PubMed]
- McNamara PJ, Laique F, Muang-In S, et al. Milrinone improves oxygenation in neonates with severe persistent pulmonary hypertension of the newborn. J Crit Care 2006;21:217-22. [Crossref] [PubMed]
- Bassler D, Choong K, McNamara P, et al. Neonatal persistent pulmonary hypertension treated with milrinone: four case reports. Biol Neonate 2006;89:1-5. [Crossref] [PubMed]
- Danhaive O, Margossian R, Geva T, et al. Pulmonary hypertension and right ventricular dysfunction in growth-restricted, extremely low birth weight neonates. J Perinatol 2005;25:495-9. [Crossref] [PubMed]


