Cardiac imaging in prosthetic paravalvular leaks
Introduction
Prosthetic paravalvular leak (PVL) is a serious complication after surgical valve replacement secondary to an inappropriate sealing of the prosthetic ring to the native cardiac tissue producing peri-prosthetic regurgitation. Although most of the cases consist on mild and asymptomatic regurgitations that are usually detected as an incidental finding, a small percentage of patients shows large and clinically significant regurgitations.
The real incidence of PVL is unknown and differs widely between different registries. PVLs are more commonly detected in mechanical valves, especially in mitral position. Paravalvular mitral leaks (PVMLs) occur in approximately 7-17% of the cases while paravalvular aortic leaks (PVALs) in 2-10% of them (1,2).
Traditionally, surgery has been the treatment of choice in symptomatic PVLs. However, percutaneous transcatheter closure is emerging as a novel and less invasive option with good outcomes in selected cases.
Echocardiography plays an essential role in the initial diagnosis, in the identification of suitable patients to undergo transcatheter intervention, and in the intra-procedure guidance.
Diagnosis of PVLs
The clinical presentation of PVLs depends on the severity of the regurgitation, varying from asymptomatic cases with mild and hemodynamically non-significant PVLs to patients with congestive heart failure (HF) and severe haemolytic anaemia secondary to large and occasionally, several PVLs.
Severe PVLs are more common in mitral than in aortic prostheses and are rarely detected in pulmonary or tricuspid position (3).
Initial diagnosis is challenging. The first signs of suspicion are the presence of an abnormal murmur in the physical examination, occasionally too soft to be detected, and the evidence of significant haemolysis in blood tests (4).
Although echocardiography is the technique of choice to identify and quantify PVL, additional imaging modalities such as, computed tomography (CT) or cardiac magnetic resonance (CMR), can be useful providing further information and confirming the disease.
Echocardiography
Echocardiography is the gold standard technique to establish the diagnosis and to assess the degree of valve regurgitation.
The first approach is commonly performed with transthoracic echocardiography (TTE). Although TTE has the potential to identify regurgitant jets through colour Doppler, it is usually limited by the presence of acoustic artefacts generated by the prosthesis that can mask the severity or even the jet. In cases of suspected clinically significant PVL, patient evaluation must be followed by a transoesophageal echocardiography (TEE) study to confirm the diagnosis.
Recently, three-dimensional (3D) TEE has shown better diagnostic accuracy compared to two-dimensional (2D) in the evaluation of leaks especially in patients with multiple defects (5). It allows a better definition of the size and shape, making this technique the gold standard for PVL evaluation. Colour-flow Doppler may be very useful to confirm that the measured area corresponds to the actual defect and that possible acoustic artefacts are not being considered as part of the orifice.
Due to the complexity of certain PVLs and the limitation of the echocardiography in certain situations (acoustic shadowing, eccentric jets), the evaluation of the PVL can be completed with other image techniques.
Additional image techniques
ECG-gated CT with 3D or four-dimensional (4D) reconstruction is a promising tool in PVLs evaluation. With retrospective ECG-gated reconstruction of helical CT acquisition sequences and 4D reconstruction it is possible to visualize the PVLs in great detail. Furthermore, it may offer the possibility of assistance during percutaneous PVL closure procedures in selected centers (6).
Angiography and CMR are additional imaging modalities. With angiography it is possible to localize and to evaluate PVL size, particularly in the aortic position. Its major challenge is the delimitation of the 3D anatomic and spatial characteristics of the leak. PVL are studied in multiples angles to document their geometry. For instance, to visualize aortic PVLs located in the right sinus of Valsalva, fluoroscopy will be orientated in the left anterior oblique view, those located in the left coronary sinus in the right anterior oblique view, and non-coronary PVLs in the lateral view. In the case of mitral PVLs, the utility of angiography is less well established; however, anterolateral PVLs are best visualized with fluoroscopy in the posteroanterior view with cranial angulation, posteroseptal PVLs in the right anterior view, and lateral PVLs in the lateral view. Clinicians should keep in mind to avoid excessive contrast administration in patients with congestive heart failure (7). CMR is able to perform accurate flow-imaging and volume-based measurements especially relevant in patients with multiple PVLs (8).
During PVL closure procedures, fluoroscopy and intracardiac echocardiography imaging provide important information. Fluoroscopy offers 2D planar image with a high degree of mobility and flexibility for oblique and angulated projections, as previously described. 2D and 3D intracardiac echocardiography (ICE) represents an alternative or a complementary technique to TEE, exclusively indicated for procedural guidance. It provides a high image resolution and detail definition. Unlike TEE, it does not require general anaesthesia, which may be especially useful for very sick patients in whom local anaesthesia may be more desirable (9).
Treatment
Medical therapy
Conventional heart failure therapy directed to afterload reduction is the first step in symptomatic patients (10). In cases with evidence of significant haemolytic anaemia, blood transfusions, erythropoietin injections and iron and folic acid supplementation may be required (11).
Surgery
Surgery has been the conventional approach of choice in symptomatic paravalvular leaks. Despite the high perioperative risk with mortality rates estimated around 16%, re-interventions have shown to improve survival compared with medical treatment at 1, 5 and 10 years (12,13).
Surgical options include the possibility of repairing the leak or prosthesis replacement depending on surgical findings related to aetiology, condition of the valve or location of the leak (14). Many techniques have been described including repair mitral leaks with direct suturing, use of patches and incorporation of healthy full-thickness autologous tissue (12).
Percutaneous therapy
Percutaneous transcatheter closure emerges as a less invasive option for high-risk patients that may reduce the morbi-mortality risk of surgical approaches.
The first procedure was made in 2003 using a ductal coil (15). Since then, numerous devices have been used (Rashkind umbrella, Amplatzer septal occluder, CardioSeal device) and recently, specifically designed devices have been developed for percutaneous paravalvular leaks closure (16).
The success of these novel techniques depends mainly on the ability to define the defect (localization, size, valve anatomy…) and 3D-TEE has demonstrated its superiority for this purpose.
Pre-procedural PVL assessment
Mitral leaks
As it was mentioned above, TTE is often performed as the initial imaging study, providing relevant information regarding chamber size and function, and evaluating the function of the prosthetic valve. Three-dimensional transoesophageal echocardiography (3D-TEE) is the most accurate technique to visualize the defect and to define its spatial characteristics: region of the defect, size and device selection (Figure 1).
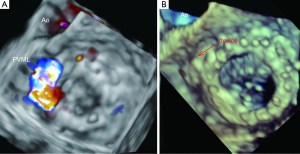
The initial TEE approach is directed to identify abnormal regurgitant jets or any other suggestive findings. Valve inspection should be performed in multiple planes sweeping the mitral prosthesis from 0º to 180º.
Leaks are defined by echocardiography as echo dropout areas outside the sewing ring confirmed by colour-Doppler. Both qualitative and quantitative echocardiographic methods can be used to characterize PVLs. The use of combined parameters is recommended. As with native mitral valves, the use of jet area and jet density can initially point out the severity in PVML. The proximal isovelocity surface area (PISA) has not been validated as a quantitative tool to estimate the severity in PVML although the presence of a large PISA shell is related with severity. The use of quantitative Doppler method is not suitable for prosthesis assessment since the antegrade transvalvular flow can be altered (17).
The use of pulsed Doppler assessment of the pulmonary vein pattern can be useful, and the detection of systolic retrograde flow is a specific sign of severe mitral regurgitation (18).
The angle at which the jet is first detected to the point of disappearance should be notice during the inspection of the valve to determine the extent of the leak. To describe the position of the leak and to facilitate the communication between the echocardiographer and the interventional cardiologist, internal landmarks such as the left atrial appendage, aortic valve and crux of the heart must be used (Figure 2) (16). Usually 3D images are orientated simulating the surgeon´s perspective, assigning the “12 o’clock” position to the anterior mitral ring at the level of the aortic valve, and “9 o’clock” at the level of the left atrial appendage.
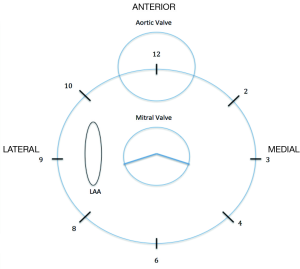
Looking for an “in face view” of the mitral prosthesis by 3D-TEE facilitate the localization of paravalvular dehiscence. 3D-TEE allows measuring the planimetry of the regurgitant orifice but the resolution may be limited when the areas of dehiscence are slit-like (19).
Aortic leaks
The assessment of aortic prosthesis offers more difficulties than mitral prosthesis due to distortion of the aortic valve plane obscured by reverberation artefacts and acoustic shadowing of the prosthesis.
The anterior feature of the aortic ring is the trickiest part to visualize because of the reverberation artefact and acoustic shadowing from the posterior valve ring. This limitation appears also with TEE, meaning that anteriorly located PVL may be difficult to characterize even despite a 3D-TEE assessment (17).
Paravalvular aortic regurgitation can be usually assessed using the following views: mid-oesophageal long and short-axis views, the transgastric view using a longitudinal imaging plane at about 100º-120º with leftward flexion and a zero-degree deep transgastric view (17).
The location of the coronary arteries remains essential. For its assessment, certain views are also recommended: the transverse plane at the aortic sinus level with the aortic root in the short axis is useful to define the left main ostium; the transverse plane at 0°-45° sweeping the aortic sinus from the annulus to the sinotubular junction; and the long-axis view of the aortic root (120º) to visualize the right coronary artery (17).
Quantification of the severity of aortic regurgitation follows the same proposed criteria than native valve regurgitation: jet width (vena contracta), jet density, jet deceleration rate, and diastolic flow reversal in the descending aorta. Guidelines suggest a semi-quantitative estimation, calculating the relationship between the jet width and the percentage of left ventricular outflow tract (LVOT) diameter: below 10% suggests mild regurgitation; 10% to 20% moderate and more than 20% severe. This method is limited by the eccentric direction and irregular shape of the defects. Vena contracta width allows an estimation of the regurgitant orifice area (EROA). This measure is limited by the radial extent of paravalvular jets and the presence of multiple minor jets (20). Jet width, jet density, jet deceleration rate and diastolic flow reversal in descending aorta are additional semi-quantitative methods to assess severity of the jet. When the AR is suspected to be more than mild, quantitative parameters such as regurgitant volume and regurgitant fraction are also suggested.
In order to describe the jet position it is also recommended to use the clockwise orientation as with the mitral valve. For instance, 5 o’clock is assigned to the commissure between the left and right coronary sinuses, 8 o’clock to the commissure between the right and non-coronary sinuses, and 11 o’clock to the commissure between the non-coronary and left coronary sinuses. Aortic PVLs are more commonly located between the right and non-coronary cusps (21).
CTA could be a good alternative to 3D TEE to identify the position of the origin of the coronary arteries.
Intra-procedure imaging to guide PVL closure
Mitral leaks
Transcatheter closure procedure is often long and technically demanding so general anaesthesia is often used. Transcatheter closure of mitral leaks is performed using either a retrograde or anterograde approach. The selection of one or another approach depends on several factors. In general, an antegrade transseptal approach combines a high degree of success in crossing the defect with the guidewire and a lower risk of bleeding complications than the retrograde trasapical route. In cases of PVLs with an anterior and lateral location the antegrade via facilitates the position of the guide wire. On the other hand, a retrograde trasapical approach provides the shortest route and is especially useful for posterior or septal PVL or multiple PVL at different locations (20).
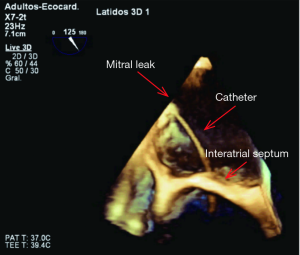
During the procedure, real time 3D-TEE offers the possibility of visualizing the location of the defect and its relation with the surrounding structures. Intracardiac thrombi must be excluded before continuing with the procedure. Zoomed 3D images allow the identification of the anatomy of the defect, helping for the selection of the most appropriate closure device. Subsequently, 3D TEE can assist the interventional cardiologist to guide the wire and catheter through the defect and to ensure adequate positioning of the catheter before device deployment (Figure 3). Once the position is considered correct, the device is deployed under echocardiographic guidance, allowing an immediate assessment of its proper seating (22).
Immediately after the device deployment, 3D-TEE allows the assessment of residual regurgitation (Figure 4). If regurgitation is still significant, device position may be adjusted or the device may be removed in order to repeat the procedure with a different device or with a combination of several devices.

Other imaging techniques as fluoroscopy and 2D-TEE can be used during the process, especially with the use of contrast but they are progressively relegated given the constant development of 3D-TEE technology.
The CT-fluoroscopy fusion imaging represents a new option especially useful in trasapical access. With this technique is possible to join the CT data obtained before the procedure (intrathoracic structures such aorta, coronary arteries, ribs, mitral prosthesis) with the live fluoroscopy in order to guide safe needle puncture (20).
Aortic leaks
Paravalvular aortic leaks are frequently corrected with a retrograde transaortic approach. As in mitral leaks, 3D-TEE and fluoroscopy are used as guidance to ensure the correct wire placement. ICE has been reported to be advantageous and safe to guide aortic leaks. ICE imaging from the right ventricular outflow tract may improve the identification of leaks located anteriorly, which are frequently challenging for TEE visualization given the acoustic shadowing of the anterior ring. Fluoroscopy may help to confirm the location and size of the leaks. They are studied in multiples angles to define their geometry. Most of these defects are located anteriorly, so the straight lateral fluoroscopic view is usually positioned with the right anterior oblique gantry to provide an orthogonal view of the prosthesis.
Echocardiography plays an important role during deployment of the device specially ensuring the normal function of the mitral subvalvular apparatus and ruling out coronary ostium obstruction.
The trasapical approach must be considered in presence of both, mitral and aortic prostheses, or an alternative when the retrograde transaortic approach has been unsuccessful (24).
Finally, prosthetic valve and device function should be assessed by TEE immediately after the device deployment. Coronary angiography may be necessary during the procedure to confirm coronary flow with the closure device in place.
Post-procedural follow-up
TTE is essential for the follow-up of prosthetic valves after paravalvular leak repair. Potential residual leaks must be detected and quantified to document stability or conversely a possible progression. Unfortunately, the same limitations previously discussed during the pre-procedure evaluation will affect the accurate detection of residual leaks in the follow up.
The use of other techniques (TEE, 3D echocardiography or CT) may be required when suspecting progression of the severity of regurgitation after repair.
Conclusions
Percutaneous intervention has become a less invasive and safe alternative over traditional surgery for the treatment of severe and symptomatic paravalvular leaks. Cardiac imaging techniques, especially 3D echocardiography, play an important role in the diagnosis and patient selection and offer a precise peri-procedural interventional guidance.
Further technical advances, an increasing interventional experience in addition to future development in imaging technology will help to consolidate this alternative option for high-risk patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hammermeister K, Sethi GK, Henderson WG, et al. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol 2000;36:1152-8. [PubMed]
- Ionescu A, Fraser AG, Butchart EG. Prevalence and clinical significance of incidental paraprosthetic valvar regurgitation: a prospective study using transoesophageal echocardiography. Heart 2003;89:1316-21. [PubMed]
- Safi AM, Kwan T, Afflu E, et al. Paravalvular regurgitation: a rare complication following valve replacement surgery. Angiology 2000;51:479-87. [PubMed]
- Skoularigis J, Essop MR, Skudicky D, et al. Frequency and severity of intravascular hemolysis after left-sided cardiac valve replacement with Medtronic Hall and St. Jude Medical prostheses, and influence of prosthetic type, position, size and number. Am J Cardiol 1993;71:587-91. [PubMed]
- Kliger C, Eiros R, Isasti G, et al. Review of surgical prosthetic paravalvular leaks: diagnosis and catheter-based closure. Eur Heart J 2013;34:638-49. [PubMed]
- Jelnin V, Co J, Muneer B, et al. Three dimensional CT angiography for patients with congenital heart disease: scanning protocol for pediatric patients. Catheter Cardiovasc Interv 2006;67:120-6. [PubMed]
- García E, Sandoval J, Unzue L, et al. Paravalvular leaks: mechanisms, diagnosis and management. EuroIntervention 2012;8 Suppl Q:Q41-52.
- Pflaumer A, Schwaiger M, Hess J, et al. Quantification of periprosthetic valve leakage with multiple regurgitation jets by magnetic resonance imaging. Pediatr Cardiol 2005;26:593-4. [PubMed]
- Deftereos S, Giannopoulos G, Raisakis K, et al. Intracardiac echocardiography imaging of periprosthetic valvular regurgitation. Eur J Echocardiogr 2010;11:E20. [PubMed]
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787-847. [PubMed]
- Shapira Y, Bairey O, Vatury M, et al. Erythropoietin can obviate the need for repeated heart valve replacement in high-risk patients with severe mechanical hemolytic anemia: case reports and literature review. J Heart Valve Dis 2001;10:431-5. [PubMed]
- Genoni M, Franzen D, Vogt P, et al. Paravalvular leakage after mitral valve replacement: improved long-term survival with aggressive surgery? Eur J Cardiothorac Surg 2000;17:14-9. [PubMed]
- Echevarria JR, Bernal JM, Rabasa JM, et al. Reoperation for bioprosthetic valve dysfunction. A decade of clinical experience. Eur J Cardiothorac Surg 1991;5:523-6; discussion 527. [PubMed]
- Al Halees Z. An additional maneuver to repair mitral paravalvular leak. Eur J Cardiothorac Surg 2011;39:410-1. [PubMed]
- Piéchaud JF. Percutaneous closure of mitral paravalvular leak. J Interv Cardiol 2003;16:153-5. [PubMed]
- Kim MS, Casserly IP, Garcia JA, et al. Percutaneous transcatheter closure of prosthetic mitral paravalvular leaks: are we there yet? JACC Cardiovasc Interv 2009;2:81-90. [PubMed]
- Zamorano JL, Badano LP, Bruce C, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur Heart J 2011;32:2189-214. [PubMed]
- Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009;22:975-1014. [PubMed]
- Becerra JM, Almeria C, de Isla LP, et al. Usefulness of 3D transoesophageal echocardiography for guiding wires and closure devices in mitral perivalvular leaks. Eur J Echocardiogr 2009;10:979-81. [PubMed]
- Kumar R, Jelnin V, Kliger C, et al. Percutaneous paravalvular leak closure. Cardiol Clin 2013;31:431-40. [PubMed]
- De Cicco G, Lorusso R, Colli A, et al. Aortic valve periprosthetic leakage: anatomic observations and surgical results. Ann Thorac Surg 2005;79:1480-5. [PubMed]
- Rodríguez Muñoz D, Lázaro Rivera C, Zamorano Gómez JL. Guidance of treatment of perivalvular prosthetic leaks. Curr Cardiol Rep 2014;16:430. [PubMed]
- Lázaro Cd, Hinojar Baydes R, Zamorano JL. Colour-flow Doppler 3D TEE showing. Asvide 2014;1:262. Available online: http://www.asvide.com/articles/275
- Kliger C, Eiros R, Isasti G, et al. Review of surgical prosthetic paravalvular leaks: diagnosis and catheter-based closure. Eur Heart J 2013;34:638-49. [PubMed]