
MicroRNA-363-3p serves as a diagnostic biomarker of acute myocardial infarction and regulates vascular endothelial injury by targeting KLF2
Introduction
Acute myocardial infarction (AMI) is a kind of serious cardiovascular disease, which is characterized by high morbidity and mortality (1). It occurs when blood flow decreases or stops to a part of the heart, leading to impairment of the heart muscle (2). The typical symptom of AMI is chest pain, which may further processes the pain in shoulder, back, neck, aim or jaw (3). It is generally considered that the development of AMI is closely associated with vascular endothelial injury and myocardial cell apoptosis (4). Endothelial cells comprise the inner layer of veins, endocardial epithelium and arteries, and are sensitive to ischemia/reperfusion injury during the development of AMI (5). Some molecules, including cardiac troponin I (cTnI), heart-type fatty acid-binding protein (H-FABP) and von Willebrand factor (vWF), have been found to release rapidly when endothelial cells were damaged, and thus are determined to be endothelial injury biomarkers (6). Various studies regarding AMI therapeutic strategies focus on the improvement of vascular endothelial function (7,8). Currently, several biomarkers have been identified for the diagnosis of AMI, including cTnI, myoglobin (MYO) and creatine kinase MB (CK-MB), and cTnI is considered the gold standard for AMI diagnosis (9,10). However, these indicators are also frequently present in some chronic coronary artery disease patients (11). Thus, novel diagnostic biomarkers with high sensitivity and specificity and new effective therapeutic strategies are urgently required to improve the treatment of AMI.
MicroRNAs (miRNAs) are a class of small noncoding RNAs with the regulatory function in gene expression at the post-transcriptional levels (12). It is reported that miRNAs are involved in various cellular processes, such as cell proliferation, migration, invasion, cell cycle and cell apoptosis (13,14). More importantly, miRNAs have been demonstrated to paly pivotal roles in the occurrence and development diverse human disease (15,16). In cardiovascular diseases, deregulated miRNAs have attracted increasing attention for their dramatically diagnostic value and therapeutic potential (17,18). In the patients with AMI, some members of miRNAs have been demonstrated to serve as diagnostic biomarkers, such as miR-208b (19) and miR-181a (20). Additionally, the therapeutic potentials of miRNAs for the treatment of AMI have also been reported (21). Increased expression of miR-363-3p has been found in myocardial infarction patients (22), and the inhibition of miR-363-3p in cardiomyocyte could suppress the hypoxia-induced cell apoptosis (23). However, the understanding of the clinical significance of miR-363 in AMI diagnosis and its effects on vascular endothelial injury remains limited.
To further improve the diagnosis and treatment of AMI, this study sought to assess the diagnostic value of miR-363-3p in patients with AMI and investigate its functional role in AMI regarding the effects on vascular endothelial injury.
Methods
Patients and serum samples
Eighty AMI patients and forty healthy individuals were included in this study. The AMI patients were collected from Shanxian Central Hospital between 2015 and 2017, and healthy controls were enrolled in the individuals who received routine physical examinations in the hospital. None of the patients had underwent any therapy before sampling. A volume of 5 mL venous blood was collected from the participants, then was centrifuged for the isolation of serum. The blood and serum samples were stored at −80 °C for further analysis. This study was performed with the approval by the Ethics Committee of Shanxian Central Hospital, each participant provided written informed consent for sample collection and use.
RNA extraction and quantitative real-time PCR (qRT-PCR)
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA in serum samples. The concentration and purity of the RNA were confirmed using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). The RNA was then reverse transcribed into cDNA using a PrimeScript RT reagent kit (TaKaRa, Shiga, Japan).
The expression of miR-363-3p was measured by qPCR, which was carried out using a 7300 Real-Time PCR System (Applied Biosystems, USA) with a SYBR green I Master Mix kit (In nitrogen, Carlsbad, CA, USA). The reaction conditions were set as follows: 95 °C for 5 min, 40 cycle of denaturation at 95 °C for 10 s, annealing at 58 °C for 15 s, and extension at 70 °C for 10 s. U6 was used as an endogenous control, and the expression values of miR-363-3p were calculated using 2−ΔΔCt method. Sequences of primer used in this analysis were as follows: miR-363-3p forward: 5'-GCCGAGAATTGCACGGTATC-3', reverse: 5'-CTCAACTGGTGTCGTGGA-3'; U6 forward: 5'-CTCGCTTCGGCAGCACA-3', reverse: 5'-AACGCTTCACGAATTTGCGT-3'.
Construction of AMI rat model
A total of 40 Sprague-Dawley rats were obtained from the Shanghai Laboratory Animal Center (Shanghai, China), with the weight of 250–350 g and an average age of 2–3 months. The rats were divided into sham group (n=10), AMI group (n=10), scrambled group (n=10) and miR-363-3p antagomir group (n=10). The rats in AMI, scrambled and miR-363-3p antagomir groups received ligation at the left anterior descending (LAD) coronary artery for AMI model construction (24). To regulate the expression of miR-363-3p in AMI animal model, the rats in scrambled and miR-363-3p antagomir groups were respectively subjected to scrambled sequence of miRNA and miR-363-3p antagomir (GenePharma, Shanghai, China) injection at 3 areas of myocardium near the LAD. In the sham group, rats only received a sham operation. All the animal experiments were performed in accordance with the Guidance for the Care and Usage of Laboratory Animals and were approved by the Animal Care and Use Committee of Shanxian Central Hospital.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of endothelial injury biomarkers, including cTnI, H-FABP and vWF, were estimated in the blood samples of the patients and rats by ELISA. ELISA was performed using an ELISA kit (Invitrogen, Carlsbad, CA, USA) following the manufacturers’ instructions.
Cell culture and transfection
Human umbilical vein endothelial cells (HUVECs) were purchased from the cell bank of the Chinese academy of sciences (Shanghai, China). The cells were cultured in endothelial growth medium at 37 °C with 5% CO2. To regulate the expression of miR-363 in HUVECs, miR-363-3p mimic, miR-363-3p inhibitor, or miR-NC (GenePharma, Shanghai, China) was transfected into the cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the protocols of manufacturers.
Cell proliferation analysis
Cell proliferation of HUVECs was estimated by MTT assay. The cells (density of 4×103 cell/well) were seeded into 48-well plates and incubated at 37 °C for 3 days. The cells were added with 0.5 mg/mL of MTT at the time points of 0, 24, 48, and 72 h, and further incubated for 4 h. After the discard of the supernatant, the cells were added with, 200 µL DMSO to dissolve the precipitate. The absorbance of the cells at 490 nm was read by an absorbance reader (BioTek Instruments, VT, USA).
Luciferase activity assay
For TargetScan, a target gene Kruppel-like factor 2 (KLF2) of miR-363-3p was predicted with the complementary sequence in its 3’-UTR. Thus, the wild type (WT) and mutant type (MT) 3'-UTR were cloned into the pMIR-Report luciferase vector (RiboBio, Guangzhou, China) to confirm the target gene. HUVECs were seeded on 12 well plates and cultured for 24 h. The pMIR-control or recombined pMIR report plasmids was co-transfected into the cells with miR-363-3p mimic, miR-363-3p inhibitor or miR-NC. The luciferase activity was measured using the Dual-Luciferase Reporter Assay (Beyotime, Jiangsu, China).
Statistical analysis
Data obtained from this study was expressed as mean ± SD and analyzed by SPSS 18.0 software (SPSS Inc., Chicago, IL) and GraphPad Prism 5.0 software (GraphPad Software, Inc., USA). Comparisons among two groups and multiple groups were, respectively, assessed by Student’s t-test and one-way ANOVA analysis followed by Tukey’s post-hoc test. Chi-square test was adopted to analyze the association between categorical variables. Spearman’s correlation test was used to assess the correlation of miR-363-3p with cTnI, MYO, and CK-MB. The diagnostic value of miR-363-3p was evaluated by the receiver operating characteristic (ROC) analysis. A P value of less than 0.05 suggested statistical significance.
Results
Clinical characteristics of the research cohort
A total of 80 patients with AMI were enrolled in this study, and 40 healthy individuals acted as healthy controls. As shown in Table 1, no significant difference was observed in the following clinical characteristics between AMI patients and healthy controls: age, gender, smoker, diabetes, total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL) (all P>0.05).
Full table
Serum miR-363-3p expression in AMI patients and healthy controls
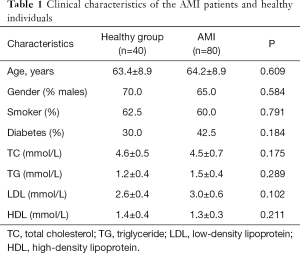
The expression of miR-363-3p in the serum samples collected from the patients and healthy individuals was estimated by qRT-PCR. From Figure 1A, we observed that miR-363-3p expression was significantly upregulated in the AMI patients compared with the healthy controls (P<0.01).
Correlation of miR-363-3p with cTnI
The concentration of cTnI, which serves the gold standard for AMI diagnosis, was also examined. As expected, the serum levels of cTnI were significantly higher in the AMI patients than that in the healthy controls (P<0.01, Figure 1B). Furthermore, we observed that the serum expression of miR-363-3p in the AMI patients was positively correlated with the concentration of cTnI (r=0.431, P<0.001, Figure 1C), indicating that miR-363-3p might also had diagnostic potential for patients with AMI.
Diagnostic value of miR-363-3p in patients with AMI
To further verify the role of miR-363-3p in AMI diagnosis, a ROC curve was plotted. The ROC curves for cTnI and miR-363-3p shown in Figure 2 revealed that the area under the curve (AUC) for cTnI was 0.923 with the sensitivity of 81.3% and specificity of 92.5% at the cutoff value of 4.040, and the AUC for miR-363-3p was 0.896 with the sensitivity of 95.0% and specificity of 62.5% at the cutoff value of 1.220.

Correlation of miR-363-3p with endothelial injury biomarkers in AMI patients
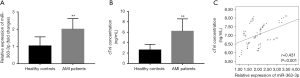
It is known that the progression of AMI is accompanied by severe endothelial injury. Thus, the endothelial injury biomarkers cTnI, H-FABP, and vWF were estimated in this study. The results in Figure 2A indicated the significant increase in the concentration of cTnI in the AMI patients compare with the healthy controls (P<0.01). Similarly, the serum levels of H-FABP and vWF were also elevated in the AMI patients compared with the healthy individuals (all P<0.01, Figure 3A,B). To determine the potential role of miR-363-3p in AMI-associated endothelial injury, the correlation between miR-363-3p and endothelial injury biomarkers was estimated. In addition to the positive correlation of miR-363-3p with cTnI shown in Figure 1C, we also found the positive correlation of miR-363-3p with H-FABP (r=0.268, P=0.016) and vMF (r=0.294, P=0.008) (Figure 3C,D), which suggested that miR-363-3p might be involved in endothelial injury in the patients with AMI.
Expression of miR-363-3p in AMI rats and its effects on AMI-induced endothelial injury
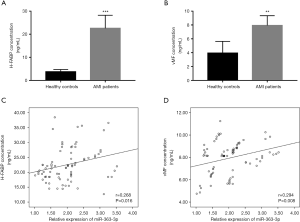
AMI animal model was constructed in this study, and the expression of miR-363-3p was also examined in the AMI rats. As shown in Figure 4A, serum levels of miR-363-3p were significantly increased after the coronary artery ligation in the AMI rats (P<0.01). To further understand the relationship between miR-363-3p and AMI-associated endothelial injury, the increased expression of miR-363-3p induced by AMI was inhibited by miR-363-3p antagomir (P<0.01, Figure 4A). According to the examination of the endothelial injury biomarker, we found that the concentrations of cTnI, H-FABP and vMF were all expected to be elevated in the AMI rats compared with the sham group, while the significant decreases were demonstrated in the serum levels of these three indicators by the knockdown of miR-363-3p in the rats (all P<0.05, Figure 4B,C,D).
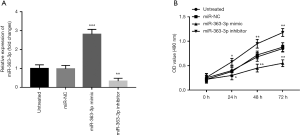
Downregulation of miR-363-3p promotes cell proliferation of HUVECs
To further investigate the effect of miR-363-3p on endothelial cell viability, cell proliferation of HUVECs was assessed. The expression of miR-363-3p in the HUVECs was promoted by miR-363-3p mimic, and was suppressed by miR-363-3p inhibitor (all P<0.01, Figure 5A). By MTT assay, we found that the overexpression of miR-363-3p in the HUVECs led to decreased cell proliferation, whereas the reduction of miR-363-3p resulted in increased cell proliferation (all P<0.05, Figure 5B).
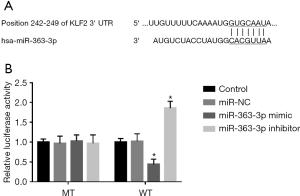
KLF2 is a target gene of miR-363-3p
This study further predicted the potential target gene of miR-363-3p and found the complementary sequence of miR-363-3p in the 3’-UTR of KLF2 (Figure 6A). To confirm this target gene, the luciferase reporter assay was conducted. The results shown in Figure 6B indicated that the luciferase activity of WT 3’-UTR of KLF2 was remarkably suppressed by the upregulation of miR-363-3p, which was enhanced by the knockdown of miR-363-3p (all P<0.05). However, there was no different in the luciferase activity of MT 3’-UTR of KLF2 between the miR-363-3p mimic and inhibitor groups (P>0.05).
Discussion
The present study investigated the clinical significance of miR-363-3p in AMI diagnosis, and explored its functional role in the progression of AMI regarding endothelial injury. The results revealed that the serum expression of miR-363-3p was increased in AMI patients and might serve a diagnostic biomarker for the identification of AMI cases from healthy individuals. In addition, a positive correlation was observed between miR-363-3p expression and endothelial injury biomarkers, including cTnI, H-FABP, and vWF, and the subsequent AMI animal analysis found decreases in these markers by the downregulation of miR-363-3p. Additionally, the knockdown of miR-363-3p was further proved to promote the cell proliferation of HUVECs. By the prediction of the target genes, KLF2 was proved to be a direct target of miR-363-3p in HUVECSs.
The dramatic dysregulation of miRNAs has attracted increasing attention for their pivotal roles in the initiation and development of various human diseases, such as tumors, metabolic diseases and cardiovascular diseases (25-27). For example, miR-17-3p was demonstrated to promote cardiac growth and myocyte proliferation, and served as a novel therapeutic target for the cardiac ischemia/reperfusion injury (28). MiR-665, as another example, has been reported to aggravate the endothelial cell apoptosis, cardiac dysfunction and coronary microvessel angiogenesis in heart failure (29). In AMI, there are also several miRNAs have been determined with critical functional roles. A study by Chen et al. revealed that overexpression of miR-133 in mesenchymal stem cells facilitated the therapeutic efficacy in the treatment of AMI (21). Protective effects of miR-125-5p were demonstrated against the AMI-induced cardiomyocyte apoptosis and impairment of cardiac structure and function (30). Upregulated miR-130 was observed in AMI, and the its inhibition in myocardial cells led to decreased myocardial injury by downregulation of PPAR-γ (31). In our study, the serum expression of miR-363-3p was found to be significantly upregulated in AMI patients than that in healthy controls, suggesting that miR-363-3p might be involved in the development of AMI.
It is known that cTnI is the gold standard for the diagnosis of AMI. Our study observed that the serum levels of cTnI were dramatically elevated in AMI patients compared with healthy controls. MiRNAs are generally considered a series of well diagnostic tools due to the specific expression pattern and stability of miRNAs in serum samples (32). Thus, the clinical significance of miRNAs has also been investigated in the diagnosis of AMI. For instance, Liu and his colleagues have identified the increased serum expression of miR-208b in AMI patients, and indicated the relative high diagnostic accuracy of miR-208b in distinction of AMI cases from unstable angina patients and healthy controls (19). Similarly, the serum elevated expression of miR-181a was also reported and played as a novel diagnostic biomarker in AMI patients (20). In the current study, a positive correlation of miR-363-3p expression with the concentration of cTnI was found, indicating the diagnostic potential of miR-363-3p in AMI. The further ROC analysis implied that serum expression of miR-363-3p had relatively high diagnostic accuracy in patients with AMI. In addition to AMI, the clinical role of miR-363-3p in diagnosis and prognosis has also been reported in adenocarcinoma of the uterine cervix (33).
In the present study, we also focused on the functional role of miR-363-3p in the regulation of AMI-associated endothelial injury. The impairment of vascular endothelium represents one of the typical phenomenon during the progression of AMI (34). A study by Liu et al. has demonstrated that downregulation of miR-92a could ameliorate AMI-induced endothelial injury by promoting cell proliferation and inhibiting cell apoptosis of endothelial cells (24). In our research cohort, we found the increases in the serum levels of endothelial biomarkers, including cTnI, H-FABP, and vWF, in AMI patients compared with healthy controls. Moreover, the expression of miR-363-3p was positively correlated with the concentrations of cTnI, H-FABP, and vWF, indicating the potential relationship between miR-363-3p and AMI-associated endothelial injury. Furthermore, the AMI rat model was constructed to confirm the effects of miR-363-3p on the development of endothelial injury, and the downregulation of miR-363-3p was demonstrated to decrease the levels of endothelial biomarkers. In addition, the cell proliferation results shown that the knockdown of miR-363-3p in human endothelial cells resulted in enhanced cell proliferation. Collectively, we considered that the reduction of miR-363-3p could ameliorate AMI by improving endothelial injury. In a published study by Meng et al. has also reported the protective effects of the inhibition of miR-363 in myocardial infarction, which were exhibited by regulation of cardiomyocyte apoptosis (23). Thus, we believed that miR-363-3p acted as a candidate therapeutic target for AMI.
To further understand the molecular mechanisms underlying the role of miR-363-3p in AMI, this study tentatively explored the target gene of miR-363-3p. KLF2 was determined as a target of miR-363-3p with the complementary sequence of miR-363-3p in its 3’-UTR. KLF2 plays a key role during angiogenesis and vascular formation (35). Notably, KLF2 and KLF4 have been reported to mediate the effects of miR-92a on endothelial cells in AMI rats (24). Therefore, we believed that the inhibition of miR-363-3p might improve AMI-induced endothelial injury by targeting KLF2. Further investigations are needed to confirm the role of miR-363-3p and the underlying mechanisms in the progression of AMI.
Conclusions
Taken together, all the data in this study revealed that the serum expression of miR-363-3p is upregulated in AMI patients and serves as a candidate diagnostic biomarker. The downregulation of miR-363-3p reduces the concentration of endothelial biomarkers and promotes the vascular endothelial cell proliferation, and this protective effect on endothelial injury may be exerted by targeting KLF2. This study provides a novel insight into the clinical and functional role of miR-363-3p in AMI, and the strategies to decrease miR-363-3p might be potential therapeutic methods for AMI treatment.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/cdt-19-700). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chien CW, Wang CH, Chao ZH, et al. Different treatments for acute myocardial infarction patients via outpatient clinics and emergency department. Medicine (Baltimore) 2019;98:e13883. [Crossref] [PubMed]
- Gupta T, Harikrishnan P, Kolte D, et al. Outcomes of acute myocardial infarction in patients with hypertrophic cardiomyopathy. Am J Med 2015;128:879-87.e1. [Crossref] [PubMed]
- Puymirat E, Aissaoui N, Bonello L, et al. Clinical outcomes according to symptom presentation in patients with acute myocardial infarction: Results from the FAST-MI 2010 registry. Clin Cardiol 2017;40:1256-63. [Crossref] [PubMed]
- Chen CL, Yang J, James IO, et al. Heparin-binding epidermal growth factor-like growth factor restores Wnt/beta-catenin signaling in intestinal stem cells exposed to ischemia/reperfusion injury. Surgery 2014;155:1069-80. [Crossref] [PubMed]
- Kong Q, Dai L, Wang Y, et al. HSPA12B Attenuated Acute Myocardial Ischemia/reperfusion Injury via Maintaining Endothelial Integrity in a PI3K/Akt/mTOR-dependent Mechanism. Sci Rep 2016;6:33636. [Crossref] [PubMed]
- Shao S, Shi Z, Tse G, et al. Effects of Trimetazidine Pretreatment on Endothelial Dysfunction and Myocardial Injury in Unstable Angina Patients Undergoing Percutaneous Coronary Intervention. Cardiol Res Pract 2019;2019:4230948.
- Tanaka S, Masuda T, Kamiya K, et al. A Single Session of Neuromuscular Electrical Stimulation Enhances Vascular Endothelial Function and Peripheral Blood Circulation in Patients With Acute Myocardial Infarction. Int Heart J 2016;57:676-81. [Crossref] [PubMed]
- Ling L, Gu S, Cheng Y. Resveratrol activates endogenous cardiac stem cells and improves myocardial regeneration following acute myocardial infarction. Mol Med Rep 2017;15:1188-94. [Crossref] [PubMed]
- Fan J, Ma J, Xia N, et al. Clinical Value of Combined Detection of CK-MB, MYO, cTnI and Plasma NT-proBNP in Diagnosis of Acute Myocardial Infarction. Clin Lab 2017;63:427-33. [Crossref] [PubMed]
- Zhang Y, Cheng J, Chen F, et al. Circulating endothelial microparticles and miR-92a in acute myocardial infarction. Biosci Rep 2017. [Crossref] [PubMed]
- Devaux Y, Vausort M, Goretti E, et al. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin Chem 2012;58:559-67. [Crossref] [PubMed]
- Zempleni J, Baier SR, Howard KM, et al. Gene regulation by dietary microRNAs. Can J Physiol Pharmacol 2015;93:1097-102. [Crossref] [PubMed]
- Jiajie T, Yanzhou Y, Hoi-Hung AC, et al. Conserved miR-10 family represses proliferation and induces apoptosis in ovarian granulosa cells. Sci Rep 2017;7:41304. [Crossref] [PubMed]
- Zhang B, Yi J, Zhang CL, et al. MiR-146a inhibits proliferation and induces apoptosis in murine osteoblastic MC3T3-E1 by regulating Bcl2. Eur Rev Med Pharmacol Sci 2017;21:3754-62. [PubMed]
- Ratnadiwakara M, Mohenska M, Anko ML. Splicing factors as regulators of miRNA biogenesis - links to human disease. Semin Cell Dev Biol 2018;79:113-22. [Crossref] [PubMed]
- Vishnoi A, Rani S. MiRNA Biogenesis and Regulation of Diseases: An Overview. Methods Mol Biol 2017;1509:1-10. [Crossref] [PubMed]
- Zhao Y, Ponnusamy M, Zhang L, et al. The role of miR-214 in cardiovascular diseases. Eur J Pharmacol 2017;816:138-45. [Crossref] [PubMed]
- Navickas R, Gal D, Laucevicius A, et al. Identifying circulating microRNAs as biomarkers of cardiovascular disease: a systematic review. Cardiovasc Res 2016;111:322-37. [Crossref] [PubMed]
- Liu X, Yuan L, Chen F, et al. Circulating miR-208b: A Potentially Sensitive and Reliable Biomarker for the Diagnosis and Prognosis of Acute Myocardial Infarction. Clin Lab 2017;63:101-9. [Crossref] [PubMed]
- Zhu J, Yao K, Wang Q, et al. Circulating miR-181a as a Potential Novel Biomarker for Diagnosis of Acute Myocardial Infarction. Cell Physiol Biochem 2016;40:1591-602. [Crossref] [PubMed]
- Chen Y, Zhao Y, Chen W, et al. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res Ther 2017;8:268. [Crossref] [PubMed]
- Bai R, Yang Q, Xi R, et al. miR-941 as a promising biomarker for acute coronary syndrome. BMC Cardiovasc Disord 2017;17:227. [Crossref] [PubMed]
- Meng X, Ji Y, Wan Z, et al. Inhibition of miR-363 protects cardiomyocytes against hypoxia-induced apoptosis through regulation of Notch signaling. Biomed Pharmacother 2017;90:509-16. [Crossref] [PubMed]
- Liu H, Li G, Zhao W, et al. Inhibition of MiR-92a May Protect Endothelial Cells After Acute Myocardial Infarction in Rats: Role of KLF2/4. Med Sci Monit 2016;22:2451-62. [Crossref] [PubMed]
- Zhang X, Chen D, Zheng J, et al. Effect of microRNA-21 on hypoxia-inducible factor-1alpha in orthodontic tooth movement and human periodontal ligament cells under hypoxia. Exp Ther Med 2019;17:2830-6. [PubMed]
- Yin S, Zhang Q, Wang Y, et al. MicroRNA-130a regulated by HPV18 E6 promotes proliferation and invasion of cervical cancer cells by targeting TIMP2. Exp Ther Med 2019;17:2837-46. [PubMed]
- Li AL, Lv JB, Gao L. MiR-181a mediates Ang II-induced myocardial hypertrophy by mediating autophagy. Eur Rev Med Pharmacol Sci 2017;21:5462-70. [PubMed]
- Shi J, Bei Y, Kong X, et al. miR-17-3p Contributes to Exercise-Induced Cardiac Growth and Protects against Myocardial Ischemia-Reperfusion Injury. Theranostics 2017;7:664-76. [Crossref] [PubMed]
- Fan J, Li H, Nie X, et al. MiR-665 aggravates heart failure via suppressing CD34-mediated coronary microvessel angiogenesis. Aging (Albany NY) 2018;10:2459-79. [Crossref] [PubMed]
- Bayoumi AS, Park KM, Wang Y, et al. A carvedilol-responsive microRNA, miR-125b-5p protects the heart from acute myocardial infarction by repressing pro-apoptotic bak1 and klf13 in cardiomyocytes. J Mol Cell Cardiol 2018;114:72-82. [Crossref] [PubMed]
- Chu X, Wang Y, Pang L, et al. miR-130 aggravates acute myocardial infarction-induced myocardial injury by targeting PPAR-gamma. J Cell Biochem 2018;119:7235-44. [Crossref] [PubMed]
- Shekari N, Baradaran B, Shanehbandi D, et al. Circulating MicroRNAs: Valuable Biomarkers for the Diagnosis and Prognosis of Gastric Cancer. Curr Med Chem 2018;25:698-714. [PubMed]
- Park H, Lee MJ, Jeong JY, et al. Dysregulated microRNA expression in adenocarcinoma of the uterine cervix: clinical impact of miR-363-3p. Gynecol Oncol 2014;135:565-72. [Crossref] [PubMed]
- Rakic M, Persic V, Kehler T, et al. Possible role of circulating endothelial cells in patients after acute myocardial infarction. Med Hypotheses 2018;117:42-6. [Crossref] [PubMed]
- Sangwung P, Zhou G, Nayak L, et al. KLF2 and KLF4 control endothelial identity and vascular integrity. JCI Insight 2017;2:e91700. [Crossref] [PubMed]


