
MicroRNA-126 promotes endothelial progenitor cell proliferation and migration ability via the Notch pathway
Introduction
Vascular endothelial dysfunction is an important initiating factor and central link in the pathogenesis of the ischemic cardio-cerebrovascular disease (ICD) (1,2). Endothelial cells (ECs) are widely acknowledged as one of the most active types of cell in the body. They are known to be involved in vascular regeneration, repair, and reconstruction, as well as coagulation and fibrinolysis, vasomotor tension, vascular smooth muscle cell proliferation, and inflammatory response (3). In vivo, ECs are rich in content, and it is of great importance that the biological function of ECs are effectively regulated.
Endothelial progenitor cells (EPCs), a type of bone marrow stem cell, and the precursor cells of ECs were first discovered in 1997. Since then, the mechanism of EPCs has been gradually clarified (4). EPCs serve an important role in angiogenesis in the embryonic stage and during angiogenesis after birth and hold potential significance for the treatment of ICD (5,6). Local vascular injury, ischemia, burns, trauma, and cytokines can stimulate EPCs to enter the blood circulation from bone marrow and migrate to the injured endothelium (7).
In basic and clinical studies, gender, age, hypertension, hyperglycemia, and other risk factors were found to affect the number and function of EPCs. Thus, it is of great significance to explore an effective and feasible intervention method to pre-treat EPCs before transplantation, to improve their number and biological function, and improve the efficacy of transplantation (8,9). Previous research has mainly focused on improving the therapeutic potential of EPCs in the final stage after they enter ischemic tissues (8). However, there have been relatively few studies on EPCs migration and the target homing process, and these were mainly limited to the local microenvironment of cerebral ischemia; the study of specific gene regulation mechanism is rare (9). Therefore, methods for EPCs migration and target homing must be improved.
In our previous studies, we found that the content of vascular endothelial growth factor (VEGF) was significantly increased in peripheral blood of ICD rats transplanted with EPCs, as well as in brain tissue from the ischemic area (10). This confirmed that the target homing mechanism of EPCs was related to the VEGF signaling pathway (10). However, the specific mechanism needs further study. With the development of genomics, microRNA-126 (miRNA/microRNA, molecule-126, and miR-126), which can specifically regulate the expression of VEGF in ECs, has gradually drawn more attention. Previous research has shown that miR-126 can be specifically expressed in the ECs of the umbilical vein (11) and that it participates in the vascular regeneration and repair process after ischemia and hypoxia. The secretion of miR-126 was found to be increased significantly in the local hypoxia microenvironment, and it could be released into the extracellular fluid through cells present in a biological fluid such as blood and cerebrospinal fluid. Recent studies have shown that the VEGF signaling pathway, which is regulated by miR-126, is involved in the regulation of vascular growth and development (12), playing an important role in maintaining cell integrity.
Therefore, this study mainly focused on exploring the effect of miR-126 on the proliferation and migration of EPCs, and the possible mechanism involved, to provide a theoretical basis for the clinical treatment with EPCs.
Methods
Preparation of bone marrow mononuclear cells (MNCs)
Ten male Sprague-Dawley (SD) rats (RGD Cat# 10395233, weighing 250–280 g) were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. All experiments were approved by the ethics research committee of Capital Medical University.
Bone marrow cells (3 mL) were extracted under sterile conditions from the distal femur of the rats using a 10 mL syringe and stored in heparinized phosphate-buffered saline. MNCs were isolated using a Ficoll gradient (Lympholite-M, Cedar lane), and the cells were cultured as previously described (10). Adherent cell culture continued for 2 weeks with the media replaced every 3 days, after which, 1×106 cells were prepared for the subsequent experiments.
Quantitative real-time PCR analysis
Total RNA was extracted from tissues and cells by Trizol reagent (Takara, Japan). RNA concentration was measured using an ultraviolet-visible spectrophotometer ultraviolet spectrophotometer (Thermo company, USA). RNA was synthesized into cDNA with a reverse transcription kit. SYBR premier ex Taq (Takara company, Japan) was used for real-time fluorescent quantitative PCR. All of these processes were carried out according to the manufacturers' instructions and the previous study (11,13).
Cell counting kit-8 (CCK8) detection of cell activity
The digested cells were resuspended in a complete medium and inoculated into 96-well plates at a concentration of 2×106/mL. After 24 h, si-miR-126 was transfected into the cells using Lipofectamine 2000, and cell viability was detected after 24, 48, 72, and 96 h, respectively. The viability of the cells was detected using CCK8 (Dojindo Laboratories company, Japan), the absorbance value was recorded at 450 nm, and the cell growth curve was drawn.
EPCs migration capability test
First, a 0.25% trypsin solution was added to digest the EPCs, according to the manufacturer's instructions. Five high-power observation fields were selected at random. The number of cells that had migrated to the bottom layer in each chamber field was counted, and the average number was calculated.
Colony formation experiment
The digested cells were resuspended and inoculated into 6-well plates at a concentration of 1×103/mL. The cells were cultured at 37 °C containing 5% CO2. After 14 d, the culture medium was discarded, and the cells were washed 3 times with PBS solution and then air-dried. Next, the cells were fixed with polyoxymethylene for 15 min and then stained with crystal violet.
Flow cytometry technology
Cells in the logarithmic growth stage were digested and resuspended, then inoculated into a 6-well plate at a concentration of 3×105/mL. Si-mir-126 was transfected into the cells using Lipofectamine 2000. After 48 h, the cells were collected and double-stained with Annexin V/PI, before being placed in a dark room for 10 min. Then, the apoptosis rate was measured by flow cytometry. After the cells were collected using the same method and conditions, they were resuspended with precooled 75% ethanol and placed overnight at −20 °C. The DNA content of the cells was detected by flow cytometry with PI staining. The time stages of the cell cycle were divided into G1/G0, S, and G2/M, and the percentage of cells at each time stage was calculated.
Transwell cell invasion assay experiment
When the fusion rate reached 80% and 90%, the si-miR-126-transfected cells were digested with trypsin, resuspended in serum-free medium at 3×105/mL, and then inoculated into a 12-well plate. After the cells were laid on the bottom of the plate, scratches were made perpendicular to the pore plate using a 1-mL pipette gun head. Then, the cell culture medium was sucked off, the pore plate was washed three times with PBS to remove the cell fragments, and serum-free medium was added. Cell migration was evaluated by measuring the cell coverage.
Western blotting to detect the expression of Notch 1
The ECs were collected, and total protein was extracted according to the manufacturer’s instructions, as previously described (14). A 30 µg protein sample underwent page electrophoresis, before being transferred to a nitrocellulose membrane for 2 h. Then, the membrane was blocked with 5% skimmed milk powder at room temperature of 25 °C for 1 h. Anti-I (1:1,000, rat anti-human Notch 1) was added, and the membrane was incubated at 37 °C. After 2 h, the membrane was washed with tris-buffered saline and incubated with secondary antibody goat anti-rat IgG (1:3,000) labeled with horseradish peroxidase, at 37 °C for 1 h. Finally, the membrane was washed with BST buffer and developed using ECL reagent. β-actin protein was used as internal reference.
Statistical analysis
All statistical analyses were carried out using SPSS 19.0. Data are presented as mean ± SD. Differences among groups were assessed by analysis of variance with Scheffe’s post-hoc test to identify individual group differences. Differences were deemed statistically significant with P<0.05 by analysis of variance and regression analysis using relevant factors. P<0.01 indicates a statistically significant difference.
Results
miR-126 expression in ECs
RT-PCR was used to detect the expression of miR-126 in the EPCs group and the EC (control) group. The relative expression of miR-126 in the EPCs group (1.91±0.21) was significantly higher than that in the control group (1.25±0.06). The difference was statistically significant (P<0.05).
Impact of miR-126 on EPCs proliferation
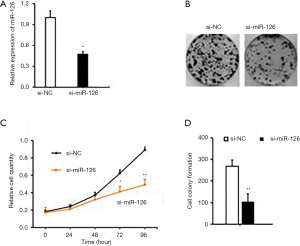
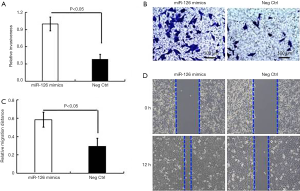
The expression level of miR-126 was significantly increased in EPCs. Therefore, we designed and synthesized three specific interference sequences of miR-126, which were transfected into EPCs, respectively, using Lipofectamine 2000, and screened out the sequence with the best interference-effect. The expression of miR-126 was significantly decreased, and the interference efficiency was higher in the two cell lines transfected with No 1. Therefore, sequence No 1 was selected for subsequent experiments (Figure 1A,B).
Then, a CCK-8 test was used to detect the proliferation of si-miR-126 cells after transfection. The results showed that the proliferation ability of si-miR-126 cells was significantly decreased compared with that of si-NC cells (P<0.01). Besides, colony formation assay was also conducted to observe the change in cell proliferation ability after si-miR-126 transfection. The results showed that the colony formation rate of EPCs was significantly lower than that of si-NCs (P<0.01) (Figure 1C,D). Based on the results of CCK-8 assay, miR-126 could promote the proliferation and cell viability of EPCs, as shown in Figure 1.
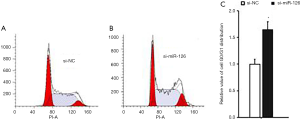
Effect of miR-126 on cell cycle and apoptosis of EPCs
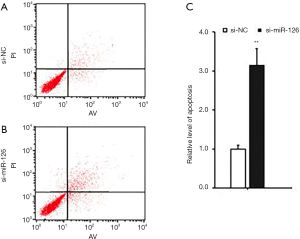
At 48 h after si-miR-126 transfection, changes in EPCs apoptosis and cell cycle were detected by flow cytometry. Compared with si-NCs, the cell cycle of EPCs in the si-miR-126 group was blocked in the G0/G1 phase (Figure 2); the experimental results showed that the apoptosis rate of cells was also significantly increased (Figure 3), and the difference was statistically significant (P<0.01). These results showed that miR-126 could regulate the cycle progression and inhibit the apoptosis of EPCs.
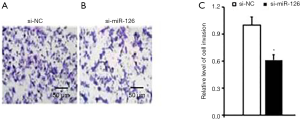
Impact of miR-126 on EPCs invasion
The effect of miR-126 on EPCs invasion was observed by the Transwell assay. At 48 h after the transfection of the miR-126 interference sequence, the number of EPCs cells passing through the chamber was significantly reduced compared with the number of si-NCs (Figure 4). This indicated that miR-126 promoted the invasive ability of EPCs.
Impact of miR-126 on EPCs migration
The effect of miR-126 on EPCs migration and invasion was observed by scratch assay. After 48 h of miR-126 interference sequence transfection, the scratch healing rate of the EPCs was significantly lower than that of the si-NCs, which was consistent with the results of the Transwell assay (Figure 5). The results showed that miR-126 promoted the migration ability of EPCs.
The activity of miR-126 in the Notch pathway
RT-PCR and Western blotting were used to observe the activity of the Notch pathway after the expression of miR-126 was disturbed. After 48 h, the mRNA and protein expressions of Notch1 and HES1 were downregulated. The expression of matrix metalloproteinase was decreased, and the abnormal activation of the Notch pathway was inhibited after the interference of miR-126 expression, which further inhibited the migration and invasion of ECs (Figure 6A).
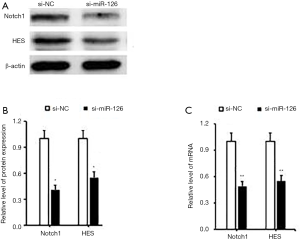
The results of Western blotting showed that compared with the control group (si-NC), the protein expression band signal of Notch 1 in the miR-126 overexpression group was weakened (P<0.05; Figure 6B). Using Image J software semi-quantitative analysis, the mRNA expression of miR-126 was 0.47±0.08 in the si-miR-126 group, and that of the si-NC group was 1. A significant difference existed between the two groups (P<0.05; Figure 6C).
Discussion
EPCs play an important role in embryogenesis and postnatal angiogenesis (5,6,15). They not only directly participate in the process of angiogenesis by differentiating into mature ECs, but also secrete a large number of cytokines to promote the proliferation and survival of ECs, thus promoting angiogenesis. EPCs could potentially be applied in the treatment of ICD (5,7). Our previous study found that EPCs from rat bone marrow could be cultured and amplified in vitro to effectively reduce acute and chronic ischemic brain damage in rats (10). However, we also found that the homing and directional migration ability of the expanded EPCs had not improved synchronously. Improvements in the capability and the efficiency of the transplanted EPCs to target the ischemic area must be addressed in the further research (15-17).
In this study, miR-126 was significantly increased in EPCs and ECs. MicroRNAs are small non-coding RNAs that make a major contribution to the regulation of cell migration, proliferation, apoptosis, and angiogenesis, and are essential for the development and progression of vascular disease (13). Previous studies have suggested that miRNAs, including miR-150 and miR-126, play a crucial role in regulating angiogenesis and migration in EPCs (18-20). These studies showed that the inhibition of miR-126 expression could block the proliferation, invasion, and metastasis of EPCs, and promote apoptosis (20). We showed that miR-126 possesses the biological functions of promoting meticulous proliferation, inhibiting apoptosis, blocking cell cycle, and promoting cell invasion and metastasis in EPCs. The results of the present study did not correspond with those of previous studies. We speculated that miR-126 regulates the related expression molecules mainly by blocking protein translation (transcriptional inhibition) or degrading mRNA (i.e., gene silencing). At least 30% of human genes are regulated by miRNA. The existing research shows that miR-126 can be specifically expressed in umbilical vein ECs (20), and it participates in the process of vascular regeneration and repair after ischemia and hypoxia. The secretion of miR-126 has been found to increase significantly in local hypoxia microenvironment and to be released through cells in the extracellular fluid such as blood and cerebrospinal fluid. Recent studies have shown that the miR-126-regulated VEGF signaling pathway is involved in the regulation of vascular growth and development (12), which plays an important role in maintaining cell integrity.
VEGF, the most important regulatory factor in angiogenesis (21), is a cell-specific angiogenic and vasculogenic mediator (22-25). It is found ubiquitously at sites of angiogenesis, and its levels are closely correlated with the spatial and temporal events of blood vessel growth (21,24). Simultaneously, it is an upstream regulator of the Notch signaling pathway and can induce the expression of Notch 1 and Dll4 (Notch ligand) in the ECs of arteries, thus promoting blood vessels development (25). Whether miR-126 can regulate the VEGF Notch signaling pathway is an important question in elucidating the mechanism of angiogenesis after ischemia. However, the effect of miR-126 on Notch signaling molecules has not been reported. In this study, we further explored its mechanisms. After inhibiting the expression of miR-126, we found that the expressions of mRNA and protein of marker molecules in the Notch pathway was decreased. This suggested that miR-126 could promote the proliferation and invasion of EPCs by regulating Notch signal transduction. It is confirmed that miR-126 regulates the target-homing mechanism of EPCs about the Notch signaling pathway, through which it may also participate in the regulation of angiogenesis. This provides an experimental basis for elucidating the new molecular mechanism of angiogenesis regulation by EPCs.
Conclusions
miR-126 is upregulated in EPCs, and the inhibition of its expression can inhibit the proliferation, invasion, and migration, as well as blocking the cell cycle and promoting apoptosis of EPCs. Therefore, the study of miR-126 provides an important theoretical basis for the clinical application of EPCs.
Acknowledgments
Funding: This work was supported by the National Key R&D Program of China 2017YFC1307500, Beijing-Tianjin-Hebei Cooperative Basic Research Program H2018206435, and the National Natural Science Foundation of China 81870935.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-178
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-178). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The experiments were approved by the ethics research committee of Capital Medical University (No. 201902024).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dávalos A, Pereira VM, Chapot R, et al. Retrospective multicenter study of Solitaire FR for revascularization in the treatment of acute ischemic stroke. Stroke 2012;43:2699-705. [Crossref] [PubMed]
- Brinjikji W, Agid R, Pereira VM. Carotid Stenting for Treatment of Symptomatic Carotid Webs: A Single-Center Case Series. Interv Neurol 2018;7:233-40. [Crossref] [PubMed]
- Fornero S, Bassino E, Gallo MP, et al. Endothelium dependent cardiovascular effects of the Chromogranin A-derived peptides Vasostatin-1 and Catestatin. Curr Med Chem 2012;19:4059-67. [Crossref] [PubMed]
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964-7. [Crossref] [PubMed]
- Minhajat R, Nilasari D, Bakri S. The Role of Endothelial Progenitor Cell in Cardiovascular Disease Risk Factors. Acta Med Indones 2015;47:340-7. [PubMed]
- Blum A, Pastukh N, Sirchan R, et al. Inhibition of endothelial Progenitor Cells Inhibition in the first 24 hours of an Acute Ischemic Cerebrovascular Event. Isr Med Assoc J 2019;21:71-6. [PubMed]
- Kiewisz J, Kaczmarek MM, Pawlowska A, et al. Endothelial progenitor cells participation in cardiovascular and kidney diseases: a systematic review. Acta Biochimica Polonica 2016;63:475-82. [Crossref] [PubMed]
- Tasev D, Koolwijk P, van Hinsbergh VW. Therapeutic Potential of Human-Derived Endothelial Colony-Forming Cells in Animal Models. Tissue Eng Part B Rev 2016;22:371-82. [Crossref] [PubMed]
- Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol 2008;45:514. [Crossref] [PubMed]
- Kong Z, Hong Y, Zhu J, et al. Endothelial progenitor cells improve functional recovery in focal cerebral ischemia of rat by promoting angiogenesis via VEGF. J Clin Neurosci 2018;55:116-21. [Crossref] [PubMed]
- Sun J, Zhang Z, Ma T, et al. Endothelial progenitor cell-derived exosomes,loaded with miR-126, promoted deep vein thrombosis resolution and recanalization. Stem Cell Res Ther 2018;9:223. Retraction in: Sun J, Zhang Z, Ma T, Yang Z, Zhang J, Liu X, Lu D, Shen Z, Yang J, Meng Q. Stem Cell Res Ther. 2019 Jun 11;10(1):162. doi: 10.1186/s13287-019-1264-3. [Crossref] [PubMed]
- Cao WJ, Rosenblat JD, Roth NC, et al. Therapeutic Angiogenesis by Ultrasound-Mediated MicroRNA-126-3p Delivery. Arterioscler Thromb Vasc Biol 2015;35:2401-11. [Crossref] [PubMed]
- Sun LL, Xiao L, Du XL, et al. MiR-205 promotes endothelial progenitor cell angiogenesis and deep vein thrombosis recanalization and resolution by targeting PTEN to regulate Akt/autophagy pathway and MMP2 expression. J Cell Mol Med 2019;23:8493-504. [Crossref] [PubMed]
- Kong Z, Shen Q, Jiang J, et al. Wogonin improves functional neuroprotection for acute cerebral ischemia in rats by promoting angiogenesis via TGF-β1. Ann Transl Med 2019;7:639. [Crossref] [PubMed]
- Zhao Y, Song J, Bi X, et al. Thymosin β4 promotes endothelial progenitor cell angiogenesis via a vascular endothelial growth factor dependent mechanism. Mol Med Rep 2018;18:2314-20. [PubMed]
- Tasev D, Koolwijk P, van Hinsbergh VW. Therapeutic Potential of Human-Derived Endothelial Colony-Forming Cells in Animal Models. Tissue Engineering Part B Reviews 2016;22:371-82. [Crossref] [PubMed]
- Zhang R, Xie X, Yu Q, et al. Constitutive Expression of Adiponectin in Endothelial Progenitor Cells Protects a Rat Model of Cerebral Ischemia. Neural Plast 2017;2017:6809745.
- Sun LL, Li WD, Lei FR, et al. The regulatory role of microRNAs in angiogenesis-related diseases. J Cell Mol Med 2018;22:4568-87. [Crossref] [PubMed]
- Wang W, Li C, Li W, et al. MiR-150 enhances the motility of EPCs in vitro and promotes EPCs homing and thrombus resolving in vivo. Thromb Res 2014;133:590-8. [Crossref] [PubMed]
- Meng Q, Wang W, Yu X, et al. Upregulation of MicroRNA-126 contributes to endothelial progenitor cell function in deep vein thrombosis via its target PIK3R2. J Cell Biochem 2015;116:1613-23. [Crossref] [PubMed]
- Liu YP, Seçkin H, Izci Y, et al. Neuroprotective effects of mesenchymal stem cells derived from human embryonic stem cells in transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab 2009;29:780-91. [Crossref] [PubMed]
- Mata-Greenwood E, Meyrick B, Soifer SJ, et al. Expression of VEGF and its receptors Flt-1 and Flk-1/KDR is altered in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2003;285:L222-31. [Crossref] [PubMed]
- Frelin C, Ladoux A, D'Angelo G. Vascular endothelial growth factors and angiogenesis. Ann Endocrinol (Paris) 2000;61:70-4. [PubMed]
- Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci 2001;114:853-65. [PubMed]
- Shifren JL, Doldi N, Ferrara N, et al. In the human fetus, vascular endothelial growth factor is expressed in epithelial cells and myocytes, but not vascular endothelium:implications for mode of action. J Clin Endocrinol Metab 1994;79:316-22. [PubMed]


