
Up-regulation of PERK/Nrf2/HO-1 axis protects myocardial tissues of mice from damage triggered by ischemia-reperfusion through ameliorating endoplasmic reticulum stress
Introduction
Acute myocardial infarction (AMI), featured by several stenosis or occlusion of the coronary artery and subsequent ischemic necrosis of cardiomyocytes, is a leading cause of death at home and abroad, accounting for an estimated 7.3 million fatalities per year (1,2). Up to now, the major clinical therapeutic methods capable of reopening the occluded artery and relieving cardiomyocytes ischemia involve thrombolysis and percutaneous coronary intervention. Yet, it is reported that these reperfusion approaches could elicit further damage to the myocardium, which is termed ischemia-reperfusion (I/R) injury (2-4). Thus, strategies responsible for improving I/R injury are also important for the treatment of AMI, which have attracted increasing attention (5-7).
Endoplasmic reticulum (ER) is reported to possess pleiotropic biological functions including protein folding and secretion, lipid metabolism, and calcium homeostasis within eukaryotic cells. Perturbations of ER homoeostasis by pathogenic factors trigger excessive misfolded or unfolded polypeptide chains accumulation in the ER, resulting in activation of specific signal pathways participating in diseases development, which is known as ER stress (ERS) (8,9). It is demonstrated that initiation and progression of I/R injury is associated with a series of complex pathophysiological processes in which ERS displays indispensable roles (10). The prolonged influx of misfolded or unfolded proteins into the ER induces activation of ERS-related signal cascades, which lead to the clearance of these abnormal proteins. Moreover, it is established that ERS activation is often accompanied by enhancement of cellular apoptosis, contributing to I/R damage in myocardial tissues (11-13). Previous studies have confirmed the expressions increase of ERS-related signaling regulators including activating transcription factor 6, calreticulin (CRT), glucose-regulated protein 78 kDa (GRP78) and C/EBP homologous protein (CHOP) in cardiomyocytes subjected to I/R intervention and agents reducing levels of these signal molecules are able to potently improve cellular death rate (14-16).
Under the physiological condition, nuclear factor erythroid 2-related factor 2 (Nrf2) locates in the cytoplasm via combining with its activity inhibitor Kelch-like ECH-associated protein 1 (Keap1). Once stimulated by damaged stresses, Nrf2 detaches from Keap1, translocate into the nucleus, and then facilitate the expression of downstream target genes like heme oxygenase-1 (HO-1), which are responsible for suppressing activation of apoptotic pathways and improving cellular survival (17). These is evidence that increased activities of Nrf2/HO-1 cascade provide protective roles for cardiomyocytes against I/R injury (11). Protein kinase-like ER kinase (PERK), a pivotal signal sensor of ERS, has been found to be reversely correlated with ERS progression (9). It is documented that PERK could induce the uncoupling of Nrf2-Keap1 complex and promote Nrf2 to migrate into the nucleus for elevating the expression of HO-1, ultimately increasing the viability of lung cells, neurons and skeletal muscle cells in response to ERS stimulation (18-20). Although up-regulation of PERK is reported to effectively alleviate cardiomyocytes damage cause by I/R process, but the underlying mechanisms has not yet been fully elucidated. Thus, in this study, we investigated whether PERK affected I/R-induced myocardial injury through improving ERS via regulating the Nrf2/HO-1 cascade.
Methods
Experimental protocol
Male C57BL/6J mice weighting 20–22 g (6- to 7-week old) were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). All animals were housed in an air conditioned room (22±2 °C) with a 12 h dark/light cycle and were free access to water and food. After one week of acclimation, mice were randomly divided into the sham and I/R groups by weight. Then the mice subjected to I/R administration were further assigned into the following groups at random: I/R, recombinant adeno-associated virus of type-9 (rAAV9)-PERK, rAAV9-Nrf2, rAAV9-HO-1, siRNA-HO-1 accompanied by rAAV9-PERK (n=10 per group).
Before the experiment, the transfection efficiency of rAAV9 in vivo was evaluated by intravenously injecting rAAV9-PERK, rAAV9-Nrf2, rAAV9-HO-1 and siRNA-HO-1 into the mice. Three days later, heart tissues were obtained for immunofluorescence and quantitative RT-PCR assay.
Mice were anesthetized by inhalation of isoflurane. The in vivo I/R model was formed by ligation of the left anterior descending (LAD) coronary artery as previous described. Briefly, after a left lateral thoracotomy, the heart was completely exposed in the intercostal space. Then 7-0 silk sutures were passed under the distal 1/3 of the LAD and tied to form an occlusion. Body temperature of mice was kept at 36.5–37 °C using an infrared temperature heater. The ligation was released after 30 min of ischemia, which was followed by reperfusion of LAD for 4 h. Sham-operated mice were injected with rAAV9-control intravenously and three days later the mice were underwent the same surgery except for the LAD coronary artery ligation.
Mice in the rAAV9-PERK, rAAV9-Nrf2 and rAAV9-HO-1 groups were injected with specific rAAV9 vectors intravenously three days before the ischemic procedures. Moreover, mice in the last group were initially transfected with siRNA-HO-1 intravenously. After 30 min, the mice were subjected to rAAV9-PERK injection. Then, three days later the mice were treated with I/R intervention. At the end of the experiment, all animals were euthanized, and the heart tissues and blood samples were collected for further analyses.
TUNEL staining
To investigate the roles of PERK expression in ameliorating myocardial apoptosis induced by I/R process, TUNEL staining was performed on paraffin sections of the cardiac tissue using commercially available kits according to the manufacturer’s protocols (Roche, Mannheim, Germany). The heart was rapidly removed and fixed in 4% paraformaldehyde solution (Servicebio, Wuhan, China) for 24 h. Then the fixed tissue was embedded in paraffin and 5 µm thick sections were made by a slicing machine. The myocardial sections were stained with TUNEL kits to label the nuclei of apoptotic cells. Under the optical microscope (Olympus, Tokyo, Japan), the nucleus of TUNEL-positive cells were stained with brown. Apoptosis index was calculated as the percent of TUNEL-positive nuclei relative to total number of nuclei and was analyzed using Image-Pro Plus 6.0 software.
Determination of serum LDH and CK-MB
Blood samples of mice were centrifuged at 3,000×g for 10 min at room temperature. Then the upper serum was obtained and used for detecting the activities of LDH and CK-MB with commercial kits according to the manufacturer’s instructions (Mlbio, Shanghai, China).
Measurement of myocardial damage
2,3,5-triphenyltetrazolium chloride (TTC) staining was used to measure the volume of myocardial infarction. Briefly, at the end of reperfusion, the LAD coronary artery was ligated again and 1% of evans blue solution (Solarbio, Beijing, China) was injected into the aortic artery to identify whether the myocardium was non-ischemic (blue staining area) or ischemic (unstained area). Afterwards, the heart was extracted from the mice quickly and frozen at −80 °C. The cardiac tissue was then sliced transversally into 2 mm thick sections, which was followed by incubation at 37 °C in 1% TTC solution (Sigma-Aldrich, USA) for 30 min away from light. After rinse and fixation, the heart section was photographed and then analyzed using Image-Pro Plus 6.0 software. The percentage of infarct size relative to ischemic size and the ratio of ischemic area to total area were measured for evaluating the severity of MI.
Quantitative RT-PCR
The total RNA of myocardial tissues was extracted using Trizol reagents (Takara, Japan). Then the total mRNA was reverse-transcribed into cDNA in accordance with the protocols provided on the Hiscript Reverse Transcriptase kit (Vazyme, China). The ABI QuantStudio6 PCR amplification instrument (Thermo Fisher, USA) and SYBR Green Master Mix (Vazyme, China) was applied to quantify PCR amplification. The primer sequences used in this study were as follows: PERK forward, 5'-CGGCAGGTCCTTGGTAATCA-3', PERK reverse, 5'-GAGGAAGTTTTGTGGGTGCC-3'; Nrf2 forward, 5'-CAGTGCTCCTATGCGTGAA-3', Nrf2 reverse, 5'-GCGGCTTGAATGTTTGTCT-3'; HO-1 forward, 5'-TTCAGAAGGGTCAGGTGTCC-3', HO-1 reverse, 5'-CAGTGAGGCCCATACCAGAA-3'; GRP78 forward, 5'-CCATCCCGTGGCATAAAC-3', GRP78 reverse, 5'-TGTCTTTTGTTAGGGGTCGTT-3'; CRT forward, 5'-CTGGTCCTTCTTCACCCCAT-3', CRT reverse, 5'-TCTGCCATGGTTCCTTTTGC-3'; CHOP forward, 5'-TCACTACTCTTGACCCTG CG-3', CHOP reverse, 5'-ACTGACCACTCTGTTTCCGT-3'; Caspase-12 forward, 5'-ATTCCTGGTGTTTATGTCCC-3', Caspase-12 reverse, 5'-TCCATTATATCTGCCTCTGC-3'; GAPDH forward, 5'-ATGGGTGTGAACCACGAGA-3', GAPDH reverse, 5'-CAGGGATGATGTTCTGGGCA-3'. The mRNA expression of target gens was normalized to GAPDH.
Western blot
Total soluble protein in cardiac tissues was extracted with RIPA lysis buffer supplemented with protease inhibitor cocktail tablets (Beyotime Biotechnology, China). The protein concentration was quantified by a BCA kit (Boster Biological Technology, China) and equal amounts of proteins were separated by electrophoresis on 10% SDS-PAGE and transferred onto the PVDF membrane, which was then rinsed and blocked with TBST buffer containing 5% bovine serum albumin for 1h at room temperature. Afterwards, the membranes were incubated overnight at 4 °C with corresponding primary antibodies against GRP78 (3177S, 1:1,000), CRT (12238S, 1:1,000), CHOP (2895S, 1:1,000) from Cell Signaling Technology (Boston, USA), caspase-12 (A0217, 1:1,000) and GAPDH (AC002, 1:5,000) from Abclonal (Boston, USA). Then membranes were washed three times with TBST and probed with secondary antibodies (Boster Biological Technology, China) for 1h. After rinse with TBST, membranes were immersed in the ECL reagent (Boster Biological Technology, China) for the visualization of protein bands. The band intensity was determined by ImageJ software. GAPDH was used as an internal standard.
Caspase-12 activity
The activity of caspase-12 in the cardiac tissue was measured by fluorometric assay kits according to the manufacturer’s instructions (BioVision, CA, USA). The protein in myocardial tissues were isolated and quantified. Then 200 µg lysate protein was applied to evaluate caspase-12 activity based on fluorometric measurement of fluorophore 7-amino-4-trifluoromethyl coumarin after cleavage from the substrates. Data of samples was obtained through a fluorescence microtiter plate reader.
Statistical analysis
The results were presented as the mean ± standard deviation. Statistical analyses were carried out using GraphPad Prim software (version 7.0, San Diego, USA). Comparisons among multiple groups were performed with one-way ANOVA followed by Tukey’s post hoc test. For all comparisons, P<0.05 was considered statistically significant.
Results
Detection of the transfection efficiency
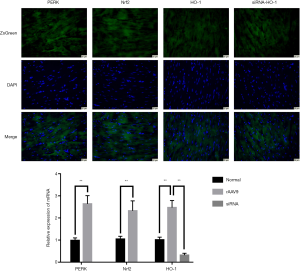
In order to evaluating the transfection efficiency of the rAAV9 in vivo, the myocardial tissues were subject to immunofluorescence and quantitative RT-PCR test. As shown in Figure 1, the ZsGreen-labeled target genes were highly expressed in the cardiac tissues. In addition, the results of quantitative RT-PCR indicated that the mRNA levels of PERK, Nrf2 and HO-1 in the heart were markedly elevated after the mice were treated with relevant rAAV9 vectors. Moreover, there was a decrease in the expression of HO-1 mRNA of cardiac tissues in the group of mice administered with the carrier loading siRNA-HO-1. These findings suggested the superior transfection efficiency of rAAV9 vectors.
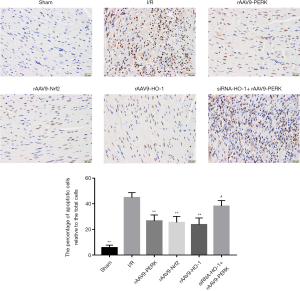
Up-regulation of PERK improved I/R-triggered myocardial apoptosis
In order to investigate the roles of PERK level increase in regulating cardiac damage induced by I/R intervention and relevant mechanisms involved, the I/R injury model was established in vivo. As shown in Figure 2, the amount of apoptotic cells in the myocardial tissue was significantly elevated in the I/R group when compared to the control group. Treatment with rAAV9-PERK effectively reduced the number of TUNEL-positive cells in the myocardium. Moreover, we found that overexpression of Nrf2 and HO-1 both decreased the apoptotic index of mice, and there was no obvious difference in apoptotic index among rAAV9-PERK, rAAV9-Nrf2 and rAAV9-HO-1 groups. In addition, after intravenous administration with siRNA-HO-1, the effects of PERK up-regulation on suppressing myocardial apoptosis induced by I/R damage were weaken, implying the pivotal roles of Nrf2/HO-1 cascade in PERK-mediated heart-protecting processes.
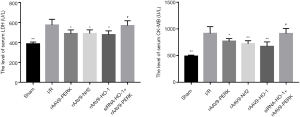
PERK/Nrf2/HO-1 pathway affected I/R-evoked release of CK-MB and LDH
For further analyzing the impacts of PERK on I/R-triggered cardiac injury, the specific enzymes associated with myocardial damage were determined. Our results indicated that I/R administration potently facilitated CK-MB and LDH efflux into the circulation, yet expression increase of PERK, Nrf2 and HO-1 effectively alleviated myocardial damage caused by I/R, as evidenced by level decrement of CK-MB and LDH (Figure 3). Additionally, we observed that under the stimulation of I/R, mice in the rAAV9-PERK group had lower content of serum CK-MB and LDH as compared to that in the siRNA-HO-1 followed by rAAV9-PERK group, which further suggested the cytoprotective effects of PERK/Nrf2/HO-1 pathway.
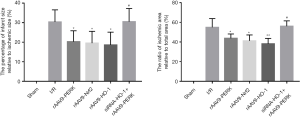
PERK overexpression markedly mitigated the severity of MI
Then, we investigated the effects of PERK on restraining the expansion of MI through TTC staining. As shown in Figure 4, administration of rAAV9-PERK markedly decreased the area of infarction and ischemia in the myocardium of mice suffering from I/R injury. Likewise, content level of Nrf2 and HO-1 reduced the volume of infarction and ischemia of cardiac tissues. Furthermore, we discovered that gene silencing of HO-1 counteracted the beneficial roles of PERK overexpression in improving MI progression.
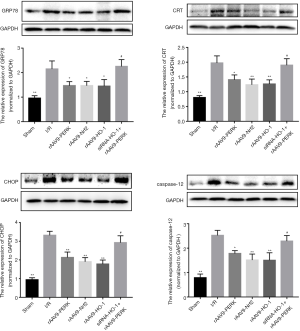
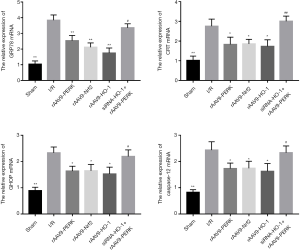
PERK-modulated Nrf2/HO-1 axis inhibited the activities of ERS-related apoptotic pathway upon I/R stimulation
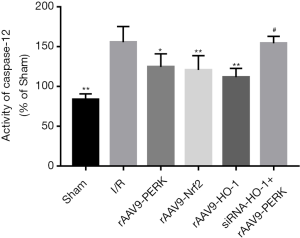
Considering that ERS displayed crucial roles in contributing to I/R injury via activating apoptosis-related cascades and PERK was capable of modulating ERS development in a negative feedback way, we explored whether increased activities of PERK/Nrf2/HO-1 pathway alleviated cardiac damage induced by I/R through disrupting the signal transduction of ERS-mediated apoptotic cascade (Figures 5,6). Our findings indicated that I/R administration potently elevated the expressions of GRP78 and CRT and the ERS-mediated pro-apoptotic factors including CHOP and Caspase-12. After treatment with rAAV9-PERK, the myocardial tissues of mice had decreased expression contents of GRP78, CRT, CHOP and Caspase-12 both at the transcriptional and translational level, which were also seen in the myocardium of mice administrated with rAAV9-Nrf2 and rAAV9-HO-1. Additionally, when mice were intravenously pretreated with siRNA-HO-1, overexpression of PERK had failed to significantly reduce the levels of signal molecules associated with ERS development and downstream apoptosis initiation, as indicated by the results of Figures 5,6. To further analyze the effects of PERK/Nrf2/HO-1 axis on the apoptotic activities, we detected the caspase-12 activity in cardiac tissues. As shown is Figure 7, I/R intervention significantly increased the pro-apoptotic property of caspase-12, yet up-regulation of PERK and downstream signal transducer Nrf2 and HO-1 prevented I/R-induced activity enhancement of caspase-12 in the heart. Moreover, expression inhibition of HO-1 markedly alleviated rAAV9-PERK-triggered activity restraint of caspase-12. These data suggested that up-regulation of PERK-regulated Nrf2/HO-1 cascade might improve I/R-triggered cardiac injury via encumbering the signaling transduction of ERS-mediated apoptotic pathway.
Discussion
Numerous laboratory and clinical studies have demonstrated that timely reperfusion therapeutics are the most effective approaches for the treatment of AMI, yet the process of reperfusion, could paradoxically, in itself, trigger additional myocardial cell death and then extend the area of MI, a phenomenon which is called I/R injury (4). There are various physiological and pathological events occurred in the initiation and progression of I/R injury and ERS has been elucidated to be a pivotal contributor to the myocardial damage process through directly inducing the activation of cardiomyocyte apoptosis-related pathways (10-13). In this study, we discovered that level increase of PERK effectively improved the cardiac injury of mice subjected to I/R intervention. Then we further investigated relevant mechanisms and found that the heart-protecting actions of PERK overexpression might be explained by the signal transduction enhancement of Nrf2/HO-1 cascade followed by activity inhibition of ERS-mediated apoptotic pathway.
When the myocardial I/R injury occurred, a number of cardiomyocytes presented apoptotic phenotypes and the size of damaged myocardium exceeded the original area of infarction and ischemia triggered by coronary artery occlusion, accompanied by specific biomarkers reflecting acute myocardial injury release into the circulation (21). Results from Liu et al. and Cao et al. report that I/R administration induced cellular death in the cardiac tissue, expand the MI size and elevate levels of blood myocardial enzymes (22,23). Similarly, our findings showed that there existed level elevations in the myocardial apoptotic index, infarction and ischemia area, circulating CK-MB and LDH of mice suffering from I/R intervention. It is well documented that PERK, which is activated by abnormal proteins accumulation in the ER, is capable of inducing translation inhibition and cell cycle arrest to facilitate the clearance of unfolded and misfolded proteins, thereby initiating the protective actions and suppressing the development of cellular apoptotic processes (9). It has been documented that activation of PERK potently promotes neuronal survival and decreases apoptosis during the early phase of intracerebral hemorrhage-induced secondary brain injury (24). Results from another study show that reactive oxygen species (ROS)-triggered death of germ cells was aggravated by PERK inhibition (25). In the present study, we found that up-regulation of PERK significantly increased cardiomyocytes survival rate and reduced serum CK-MB and LDH levels of mice damaged by I/R processes, suggesting that PERK was beneficial for improving I/R-induced myocardial injury.
Cumulative evidence indicates that Nrf2 is a transcription factor directly participating in improving various pathogenic processes including inflammation, oxidative stress and ERS and maintaining the intracellular homeostasis. When cells are exposed to pathological factors, Nrf2 migrates from the cytoplasm into the nucleus, and binds to the antioxidant response element in the upstream promoter region of protective genes like HO-1, thus improving the anti-apoptotic effects of cells (17,26). Previous studies have demonstrated that PERK exerts protective effects against cell death through regulating specific downstream signal cascades and Nrf2/HO-1 is an important pro-survival pathway of them. Yamada et al. report that Boron ameliorates DNA damage and cell death via activating PERK/Nrf2 signal cascade (27). Fujiki et al. show that the potential mechanisms underlying tolvaptan alleviates ROS-induced chronic kidney injury are ascribed to the up-regulation of PERK-dependent Nrf2/HO-1 signaling transduction (28). Moreover, it has been indicated that Nrf2/HO-1 axis is a direct PERK substrate and effector of PERK-mediated lung cell survival upon bleomycin stimulation (18). In this study, we found that the I/R-triggered cardiomyocyte apoptosis and CK-MB and LDH release was reduced in mice treated with rAAV9-PERK, which was also seen in mice administered with rAAV9-Nrf2 and rAAV9-HO-1. In addition, our results showed that I/R-induced myocardial injury was more severe in mice treated with siRNA-HO-1 followed by rAAV9-PERK injection when compared to mice infected with rAAV9-PERK alone, which suggested that up-regulation of PERK-dependent Nrf2/HO-1 signal flow was responsible for the mitigation of cardiac damage induced by I/R intervention.
It has been well-established that ERS is a pivotal pathophysiological process deeply involved in the initiation and progression of cardiovascular disease including atherosclerosis, heart failure and hypertension (8,9). Recently, ERS is found to be capable of participating in the development of I/R-induced myocardial injury following AMI through regulating cellular apoptotic-related actions, which has been verified by a series of evidences: ERS-related signal molecules are increased in the I/R environment, leading to the activation of downstream caspase-12 required for apoptosis initiation (29,30). Similarly, our results suggested that there was a level elevation of GRP78, CRT, CHOP and caspase-12 in the cardiac tissues of mice subjected to I/R administration. It is now recognized that agents possessing inhibitory effects on ERS development are able to alleviate the myocardial damage triggered by I/R process. For instance, calcium-sensing receptor and nobiletin are reported to effectively ameliorate I/R-evoked cardiac injury via improving ERS-associated apoptosis, as seen by expression reduction of GRP78, CHOP and caspase-12 (14,15). Considering the crucial roles of ERS in I/R-induced cellular death, we investigated whether PERK-mediated Nrf2/HO-1 cascade affected the development of ERS in this study. The results of molecular detection showed that I/R intervention-induced expression increase of GRP78, CRT, CHOP and caspase-12 in the myocardial tissue was significantly suppressed when mice were intravenously injected with rAAV9-PERK, -Nrf2 or -HO-1. Moreover, we discovered that expression inhibition of HO-1 invalidated the roles of PERK overexpression in restraining signal transduction of ERS-mediated apoptotic cascade. Additionally, I/R-evoked elevation of the pro-apoptotic activity of caspase-12 was repressed by enhanced signaling transduction of PERK/Nrf2/HO-1 axis. Thus, our findings suggested that the potential mechanisms by which up-regulation of PERK-dependent Nrf2/HO-1 signal flow attenuated I/R-triggered cardiac damage might be attributed to the inhibition of ERS-regulated apoptotic activities.
Conclusions
In conclusion, our findings shows that up-regulation of PERK-mediated Nrf2/HO-1 pathway significantly improves the myocardial injury of mice with I/R intervention. We, for the first time, discover that the protective actions of PERK/Nrf2/HO-1 pathway against I/R damage might be explained by the expression reduction of ERS-related signal transduction factors, followed by inhibition of downstream apoptotic activities. Our results provide the evidence that up-regulation of PERK/Nrf2/HO-1 has the potential to serve as an effective approach for the treatment of I/R-induced myocardial injury in the clinical practice.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81500274 and 81600288), the Natural Science Foundation of Hubei Province (No. 2015CFB207), the Fundamental Research Funds for the Central Universities (No. 2042015kf0068).
Footnote
Data Sharing Statement: Available at: http://dx.doi.org/10.21037/cdt-20-12
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at: http://dx.doi.org/10.21037/cdt-20-126). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This animal study was performed in strict accordance with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health. The experimental procedures were approved by the Animal Care and Use Committee of Renmin Hospital of Wuhan University. Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mendis S. Global progress in prevention of cardiovascular disease. Cardiovasc Diagn Ther 2017;7:S32-8. [Crossref] [PubMed]
- Wider J, Przyklenk K. Ischemic conditioning: the challenge of protecting the diabetic heart. Cardiovasc Diagn Ther 2014;4:383-96. [PubMed]
- Zhou H, Wang J, Zhu P, et al. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2α. Basic Res Cardiol 2018;113:23. [Crossref] [PubMed]
- Vogel B, Mehta SR, Mehran R. Reperfusion strategies in acute myocardial infarction and multivessel disease. Nat Rev Cardiol 2017;14:665-78. [Crossref] [PubMed]
- Fordyce CB, Gersh BJ, Stone GW, et al. Novel therapeutics in myocardial infarction: targeting microvascular dysfunction and reperfusion injury. Trends Pharmacol Sci 2015;36:605-16. [Crossref] [PubMed]
- Heusch G. 25 years of remote ischemic conditioning: from laboratory curiosity to clinical outcome. Basic Res Cardiol 2018;113:15. [Crossref] [PubMed]
- Bøtker HE, Hausenloy D, Andreadou I, et al. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol 2018;113:39. [Crossref] [PubMed]
- Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res 2010;107:1071-82. [Crossref] [PubMed]
- Liu MQ, Chen Z, Chen LX. Endoplasmic reticulum stress: a novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol Sin 2016;37:425-43. [Crossref] [PubMed]
- Qi D, Young LH. AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab 2015;26:422-9. [Crossref] [PubMed]
- Cominacini L, Mozzini C, Garbin U, et al. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radic Biol Med 2015;88:233-42. [Crossref] [PubMed]
- Yan M, Shu S, Guo C, et al. Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury. Ann Med 2018;50:381-90. [Crossref] [PubMed]
- Folch-Puy E, Panisello A, Oliva J, et al. Relevance of endoplasmic reticulum stress cell signaling in liver cold ischemia reperfusion injury. Int J Mol Sci 2016;17:807. [Crossref] [PubMed]
- Liu C, Li H, Zheng H, et al. CaSR activates PKCδ to induce cardiomyocyte apoptosis via ER stressassociated apoptotic pathways during ischemia/reperfusion. Int J Mol Med 2019;44:1117-26. [PubMed]
- Zhang BF, Jiang H, Chen J, et al. Nobiletin ameliorates myocardial ischemia and reperfusion injury by attenuating endoplasmic reticulum stress-associated apoptosis through regulation of the PI3K/AKT signal pathway. Int Immunopharmacol 2019;73:98-107. [Crossref] [PubMed]
- Wang S, Bian W, Zhen J, et al. Melatonin-mediated Pak2 activation reduces cardiomyocyte death through suppressing hypoxia reoxygenation injury-induced endoplasmic reticulum stress. J Cardiovasc Pharmacol 2019;74:20-9. [Crossref] [PubMed]
- Loboda A, Damulewicz M, Pyza E, et al. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 2016;73:3221-47. [Crossref] [PubMed]
- Lee EJ, Cardenes N, Alvarez D, et al. Mesenchymal stem cells reduce ER stress via PERK-Nrf2 pathway in an aged mouse model. Respirology 2020;25:417-26. [Crossref] [PubMed]
- Huang T, Zhao J, Guo D, et al. Curcumin mitigates axonal injury and neuronal cell apoptosis through the PERK/Nrf2 signaling pathway following diffuse axonal injury. Neuroreport 2018;29:661-77. [Crossref] [PubMed]
- Zhang J, Wei Y, Qu T, et al. Prosurvival roles mediated by the PERK signaling pathway effectively prevent excessive endoplasmic reticulum stress-induced skeletal muscle loss during high-stress conditions of hibernation. J Cell Physiol 2019;234:19728-39. [Crossref] [PubMed]
- Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol 2016;13:193-209. [Crossref] [PubMed]
- Liu AJ, Pang CX, Liu GQ, et al. Ameliorative effect of sevoflurane on endoplasmic reticulum stress mediates cardioprotection against ischemia-reperfusion injury. Can J Physiol Pharmacol 2019;97:345-51. [Crossref] [PubMed]
- Chang P, Zhang M, Zhang X, et al. B-type natriuretic peptide attenuates endoplasmic reticulum stress in H9c2 cardiomyocytes underwent hypoxia/reoxygenation injury under high glucose/high fat conditions. Peptides 2019;111:103-11. [Crossref] [PubMed]
- Zhang J, Zhang P, Meng C, et al. The PERK pathway plays a neuroprotective role during the early phase of secondary brain injury induced by experimental intracerebral hemorrhage. Acta Neurochir Suppl 2020;127:105-19. [Crossref] [PubMed]
- Zhang G, Yang W, Jiang F, et al. PERK regulates Nrf2/ARE antioxidant pathway against dibutyl phthalate-induced mitochondrial damage and apoptosis dependent of reactive oxygen species in mouse spermatocyte-derived cells. Toxicol Lett 2019;308:24-33. [Crossref] [PubMed]
- Wu Z, Zai W, Chen W, et al. Curdione ameliorated doxorubicin-induced cardiotoxicity through suppressing oxidative stress and activating Nrf2/HO-1 pathway. J Cardiovasc Pharmacol 2019;74:118-27. [Crossref] [PubMed]
- Yamada KE, Eckhert CD. Boric acid activation of eIF2α and Nrf2 is PERK dependent: a mechanism that explains how boron prevents DNA damage and enhances antioxidant status. Biol Trace Elem Res 2019;188:2-10. [Crossref] [PubMed]
- Fujiki T, Ando F, Murakami K, et al. Tolvaptan activates the Nrf2/HO-1 antioxidant pathway through PERK phosphorylation. Sci Rep 2019;9:9245. [Crossref] [PubMed]
- Guo J, Bian Y, Bai R, et al. Globular adiponectin attenuates myocardial ischemia/reperfusion injury by upregulating endoplasmic reticulum Ca(2)(+)-ATPase activity and inhibiting endoplasmic reticulum stress. J Cardiovasc Pharmacol 2013;62:143-53. [Crossref] [PubMed]
- Shen D, Chen R, Zhang L, et al. Sulodexide attenuates endoplasmic reticulum stress induced by myocardial ischaemia/reperfusion by activating the PI3K/Akt pathway. J Cell Mol Med 2019;23:5063-75. [Crossref] [PubMed]


