
Hybrid transcatheter left ventricular reconstruction for the treatment of ischemic cardiomyopathy
Introduction
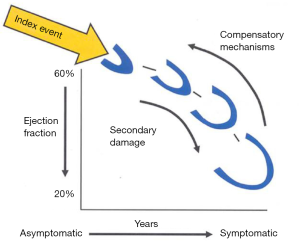
Heart failure (HF) due to systolic left ventricular (LV) dysfunction is one of the major causes of mortality and morbidity the western world (1), with ischemic etiology being observed as the most common. LV enlargement, determining an increased end-systolic volume (ESV) and end-diastolic volume (EDV) is the pathophysiological mechanism to accommodate the decreased LV systolic performance in the medium-to-long term after an index event (either an acute or a slowly progressive one, Figure 1) that disrupts the pumping function of the heart (2). This mechanical adaptation to declining systolic function, termed as LV remodeling, aims at preservation of stroke volume by recruiting the Frank-Starling reserve, but eventually turns in overt HF when the LV chamber reaches the most rightward end of the pressure/volume relationship, where high filling pressure causes congestive symptoms and failure to maintain cardiac output ensues. It has been shown in literature that both pharmacological and non-pharmacological treatments such as cardiac resynchronization therapy, that reduce cardiac volume, either ESV or EDV, can significantly improve mortality in HF patients with LV systolic dysfunction, the largest volume reduction being associated with greater advantage in survival (3,4).
Surgical techniques in ischemic cardiomyopathy
Surgical ventricular reconstruction (SVR)
Ischemic cardiomyopathy due to myocardial infarction with extensive LV scarring or aneurysm formation is a distinct and well-known model of LV remodeling that occurs in nearly 20% of patients despite state-of-the-art interventions aiming at early coronary revascularization and optimal medical treatment. The maladaptive LV dilation due to extensive scarring, including aneurysm formation, promotes several other adverse structural changes in LV geometry, like increased LV wall tension and mechanical inefficiency of the viable ventricular segments, papillary muscle displacement and onset of significant functional mitral regurgitation (FMR), and represents the substrate for ventricular tachycardia (VT).
The new imaging modality which allows tissue characterization—namely magnetic resonance (MR) scanning—has enabled semi-quantitative estimation of LV scar burden, that is closely correlated to HF occurrence: advanced HF occurs in >50% of patients showing scarring of >50% of the LV perimeter according to the European Society of Cardiology criteria (5). Differently from non-ischemic LV enlargement, the presence of extensively scarred LV areas or of an aneurism offers a unique possibility to achieve reverse remodeling by surgical reconstruction of the left ventricle (SVR) (6-8).
Outcome
Tailoring the surgical reconstruction to achieve an appropriately-sized LV volume has proven the safety and efficacy of this approach in patients with HF following myocardial infarction causing extensive LV scarring: in the non-randomized RESTORE group experience, 5-year survival of patients with an average left ventricular ESV index (LVESVi) of 76 mL/m2 was 68% with 85% of patients in NYHA class I and II (preoperatively 67% of patients were in NYHA 3 and 4), in-hospital mortality being 6.6% (9). In that study, concomitant coronary grafting occurred in 95% of patients and mitral repair in 22%, this latter being more common in patients with an LVESVi ≥80 mL/m2 and in those with a left ventricular ejection fraction (LVEF) ≤30% (9). This combination of surgical procedures, i.e., ventricular, coronary and valve surgery, highlights the complexity of pre-operative patient’s characterization.
HF due to ischemic etiology stands on several pathophysiological factors that need to be evaluated in each individual patient to achieve tiered therapy. Indeed, the prevalence of large akinetic but viable myocardial segments suggest coronary revascularization as a curative treatment, whereas large scarred areas suggest ventricular surgery. Thus, comprehensive evaluation of HF patients of ischemic etiology shall assess: (I) LV volume and geometry; (II) extent of myocardial viability; (III) extent of scarred LV myocardium; (IV) coronary artery anatomy; (V) FMR and its pathophysiology. In this perspective, the interpretation of the single randomized trial comparing coronary revascularization alone [coronary artery bypass grafting (CABG)] vs. CABG + SVR in patients with ischemic heart disease and systolic LV dysfunction [ejection fraction (EF) <35%] needs to be taken cautiously for several reasons: only half of the population had ≥ NYHA 3; the modality of SVR, that did not specify tailoring of LV volume reduction as to improve simultaneously systolic and diastolic function as in the Dor procedure (10,11); the certification of the surgeons to perform SVR allowed moderate-volume operators ; the end-point of volume reduction to be achieved was not pre-specified (8). Indeed, the sub-analysis of the STICH trial itself clearly pointed to a survival benefit in patients who had SVR and obtained a LVESVi ≤60 mL/m2 at follow-up [hazard ratio (HR) =0.33], or a ≥30% reduction from baseline. Insights from the STICH trial confirm the complex interplay between baseline LV remodeling (LVESVi) and viable myocardium that is susceptible of contractile recovery thanks to revascularization: though the extent of LVESVi improvement was more likely in patients with the largest ventricle (LVESVi ≥90 mL/m2) either by CABG alone or CABG + SVR, the magnitude of effect was greater with SVR, and a clear survival benefit appeared in those patients with baseline LVESVi between 60 and 90 mL/m2 (12).
These observations have several implications, that better spotlight the role of SVR in the management of HF patients: while little benefit has to be expected in those patients with limited akinetic or bulging LV segments and a preoperative LVESVi <70 mL/m2, an extensively enlarged and remodeled ventricle (baseline LVESVi >90 mL/m2) might limit the benefit of SVR on survival at long term due to a more advanced stage of the disease (12). Thus, comprehensive patients’ evaluation and timely intervention are key to improve the outcome of patients with HF/systolic dysfunction due to ischemic heart disease.
Despite substantial evidence, SVR has declined in the past decade often owing to misperception of the survival benefit compared its short- and mid-term risks (7-10), as reported in the STICH trial (9,12). Consistently with this scenario, a new technique for SVR without ventriculotomy by means of the Revivent™ system (Bioventrix Inc., San Ramon, CA, USA), thus avoiding extracorporeal circulation and minimizing the postoperative recovery of LV function, was developed (13).
Hybrid left ventricular reconstruction
The Revivent™ system
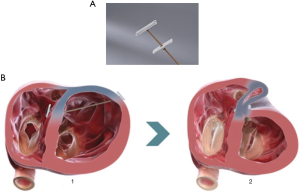
The Revivent™ system enables a minimally invasive ventricular reconstruction by an off-pump procedure without ventriculotomy: this term comprises two different procedures, hybrid transcatheter ventricular reconstruction (HVR), and epicardial Revivent™. Ventricular reconstruction is indeed achieved either as a hybrid procedure (trans-jugular vein approach combined to left thoracotomy) performed by a heart team (interventional cardiologists and cardiac surgeons) achieving LV plication by anchor pairs (one endoventricular and one epicardial for each pair, but for the apical pair), or as a purely epicardial LV plication done by cardiac surgeons (Epicardial Revivent™). The earliest experience was the Epicardial Revivent™, being carried out by Wechsler et al. (13) on 31 consecutive patients, who showed that ventricular reconstruction can be achieved without ventriculotomy in patients with large antero-septal scars due to previous myocardial infarction by apposition of the scarred free LV wall to the septal side of the scar with serial paired anchors (23 mm × 4 mm) placed through epicardial transmural tethers (Figure 2, panel A), excluding the non-viable portions of the chamber. The evolution of the system led to HVR, a transcatheter modification of the procedure that enables a larger LV volume reduction, by plication of the anterior and free wall LV scar against the right ventricular (RV0 septal scar of anteroseptal infarctions (Figure 2, panel B) by means of titanium anchor pairs (the internal one hinged, the external one is the locking anchor) covered by polyester coating (Figure 2A). The anchor pairs are connected to each other by a tether (1.7 mm × 1.0 mm) made of poly-ether-ether-ketone (Figure 2A). This hybrid transcatheter approach is a HVR, that involves two teams working together, cardiac surgeons and interventional cardiologists, and is accomplished via a left anterolateral thoracotomy for the placement of epicardial anchors, plus a trans-jugular endoventricular anchor placement against the RV septal scar (Figure 3A). The distance between anchors is adjustable and is determined by the location of the sliding locking anchor relative to the fixed hinged anchor (Figures 2,3). Anteroseptal scarred myocardium is excluded by drawing the locking (epicardial) and the hinged (from the right side of septum) anchors together. Once the hinged anchor has been deployed against the scarred tissue of the RV septum, the locking one is drawn towards it over the tether by a force gauge to control compression (Figure 2B). The action is repeated by adding paired anchors until a linear portion of the anterolateral wall is in contact with a corresponding portion of the septum, thus excluding a discrete length of the LV wall from the circumference of the chamber (Figure 3B). The apical pair of anchors is purely placed epicardially by the surgeon, owing to their proximity (Figure 3B) and to the direct visualization of the apex by the surgeon. Thus, a sizeable portion of the LV circumference and LV volume is reduced primarily due to decreased circumference and radius. Owing to the very limited thrombogenic potential of the anchors, patients are treated with warfarin for 3 months after surgery, followed by aspirin thereafter.
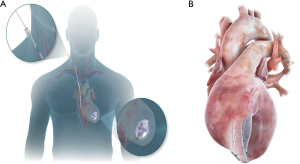
HVR is based on advanced imaging and is best suited for a hybrid room rather than for a conventional operating room (14-16). In fact, high quality radioscopy is needed to guide crossing the LV cavity and puncture through the anterior RV septum, to enable passing a guidewire that is snared inside the main guiding catheter located in the RV, thus providing a working station to deliver the hinged anchor to the septum and the tether to the LV epicardial surface. Transesophageal echocardiography (TE) is key to assist the snaring procedure and anchor deployment against the RV septum, to ensure that no interaction with tricuspid valve structures occurs. As such, the procedures require a high level of interaction amongst cardiac surgeon, cardiologist and TE specialist, with continuous communication being the customary operational modality.
Patient selection for HVR
Patients selection follows the same approach of a SVR procedure, in that a comprehensive evaluation of patients with systolic dysfunction and HF is needed: (I) previous CABG or other cardiac surgery is a contraindication owing to graft presence and adherences; (II) FMR extent and pathophysiology are investigated, as grade 4 mitral regurgitation patients were not included in the studies so far (13-17); (III) evaluation of myocardial ischemia that can be corrected is mandatory; (IV) patients with unstable VT need to be treated by drugs and/or VT ablation before the procedure.
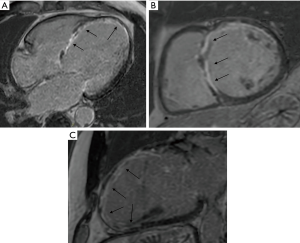
The selection process is of the outmost importance, as the anchors need to be located on scarred tissue to avoid penetration in the myocardium resulting in a ventricular septal defect during the endoventricular anchor placement. Thus, the screening process relies on pre-operative gadolinium-enhanced MR scanning to measure LV volume, LV thickness, presence of non-stratified/recent LV thrombus, RV function, and to locate anterolateral and septal transmural scar (Figure 4). Scar transmurality is key to avoid ventricular perforation when force is applied on the epicardial anchor, so a minimum 50% scar transmurality on the RV septal side is mandatory for the procedure (Figure 4). In the absence of septal scar, a purely epicardial procedure can be considered (13). Echocardiography is also needed to assess preoperatively FMR, regional wall contractility, RV function, tricuspid regurgitation; when MR scan is not feasible for any reason, or imaging is suboptimal due to implantable cardioverter-defibrillator (ICD)/pacemaker, echocardiography and computed tomography (CT) scan are used for the screening process. The key points of patients’ selection process are summarized in Table 1.
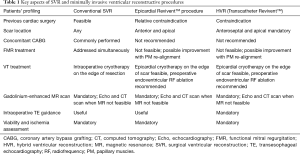
Full table
Outcome
Outcome of minimally invasive ventricular reconstruction (Epicardial Revivent™ + HVR)
By means of the pioneering non-ventriculotomy off-pump SVR adopted by Wechsler et al. (13) with the Epicardial Revivent™, a durable reduction of LVESVi (−39.6%±14.8%) and of LVEDVi (−32.2%±14.9%) was achieved at 1-year follow-up. This approach enables substantial volume reduction via a totally epicardial procedure, and was adopted in a total 52 patients confirming its safety and efficacy (17). As of October 2019, ventricular reconstruction by the Revivent™ system had been used in 203 patients, of which 52 received the epicardial procedure and 151 the HVR one (16,17). In the pioneering experience leading to CE mark, in hospital mortality was 4.5% (4 patients), with a 90% 12-month survival (16), that compares favorably with historical data of SVR studies, namely the STICH and the RESTORE populations (7,9). Baseline ESVi was comparable to the RESTORE population, and decreased by 27% on average at 12-month follow-up (P<0.001), while EDVi decreased by 24% on average (P<0.0001) (16). LVEF had an absolute 5% increase compared to baseline (P<0.005) (16). There was a significant improvement of NYHA class and of the Minnesota Living with HF Questionnaire, an average 53-m increase at 6-minute walking test, and strikingly low (13%) re-hospitalization rate at 6 months (16). However, as experience and adoption of the HVR increased, instead of the Epicardial Revivent™ procedure, the results tended to improve in the past 2 years, with superior decrease of LV volume and minimal serious complications (<1% mortality at hospital discharge), as reported in the post-market registry (Figure 5).
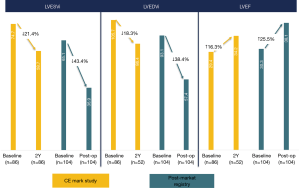
Discussion
HVR is a promising treatment for HF patients with large anteroapical LV akinetic scars or aneurysms due to previous myocardial infarction, who may benefit from LV volume reduction. The extent of LV volume reduction by the Revivent™ procedure (both epicardial and HVR) seems comparable to SVR, with a favorable clinical outcome at follow-up (Figure 5). Owing to the less invasive nature of HVR, it seems reasonable that it will gain popularity over SVR, whose application has been declining in the past decade. Given the relative novelty of this approach, survival rates and LV functionality data at very long follow-up (10) are not available, while 5-year mortality and LV remodeling data from the earliest series are being collected to confirm the efficacy of this technique. Though the size of the population is limited compared to the main SVR series, a smaller baseline ESVi and a better LVEF were not associated to better outcome (mortality and reverse remodeling) in the HVR experience as in the STICH trial (12), possibly due to absence of severe postoperative events that may be related to extracorporeal circulation. This may suggest that severe LV enlargement is not a contraindication for an HVR procedure, once the clinical conditions have been optimized. Indeed, the patients’ profile in the Revivent™ system CE mark study (16) was similar to SVR studies but for the extent of grade 4 mitral regurgitation, that accounted on average for 20% of patients enrolled in SVR studies (7-9), whereas it was a contraindication in Revivent™ procedures (13-17). In this perspective, SVR stands as the preferred surgical approach to patients who can benefit by the treatment of mitral regurgitation. It has to be noted, however, that patients with grade 2 and 3 FMR were treated by HVR: 63% had at least 1 grade decrease of FMR, whereas it was unchanged in the other 37%. This observation is expected, owing to the restoration of LV geometry that occurs by LV volume reduction, causing less papillary muscle displacement, at least partial recovery of LV torsion dynamics, and myofibers re-orientation (18). From a morphological perspective, the LV geometry appears less spherical and more conical after HVR, with a decreased FMR in selected patients (Figure 6). The absence of ventriculotomy and extracorporeal circulation may contribute to a lower in-hospital mortality and a faster recovery for patients undergoing HVR compared to SVR, however mandates a careful management of severe patients, whose hemodynamics needs to be optimized preoperatively. Specific attention is also placed to pre-operative assessment of ventricular arrhythmias, whose onset might otherwise threaten severe instability during the HVR procedure: the majority of these patients are vulnerable to monomorphic VT and are very often ICD recipients. Those patients with recently documented non-sustained VT or ICD therapy delivery are at the highest risk of arrhythmia recurrence. Besides pharmacologic treatment, transcatheter VT ablation is nowadays applied with a high success rate in preventing VT recurrences, though 30–40% of patients have relapses. The management of patients with recent ICD therapy delivery or with residual VT after catheter ablation is very debatable, SVR yielding very good results thanks to intraoperative cryoablation (7,8,10): in such a patient profile, SVR coupled to intraoperative VT ablation should be considered as a truly comprehensive approach to the patient.
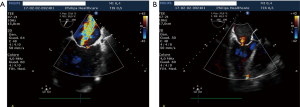
Conclusions
The HVR procedure seems a promising treatment for HF patients who may benefit from LV volume reduction at very reasonable mortality and with good results at follow-up. A controlled study on top of optimal medical treatment is warranted to confirm its role in the management of HF patients. Limitations are to be acknowledged, especially the limited number of patients treated for a shorter follow-up compared to SVR. Unlike SVR, FMR is not a target of the procedure, but significant improvement may be achieved when FMR is mainly related to the ventricular disease rather than to the valve structures. In this respect, minimally invasive hybrid treatment of both the ventricular and valvular disease may be foreseen in the future (19,20), to target all the pathophysiologic contributors of LV dysfunction and HF.
Acknowledgments
This paper was presented in the joint session of the 27th Annual Meeting of the ISMCS 2019 and RHICS 17th Expert Forum, October 21-23, 2019, Bologna, Italy.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Roland Hetzer) for the series “Heart Failure in the Young and Old: Insights into Various Therapies” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-265). The series “Heart Failure in the Young and Old: Insights into Various Therapies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Mann DL. Mechanisms and models in heart failure: A combinatorial approach. Circulation 1999;100:999-1008. [Crossref] [PubMed]
- Kramer DG, Trikalinos TA, Kent DM, et al. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol 2010;56:392-406. [Crossref] [PubMed]
- Ypenburg C, van Bommel RJ, Borleffs CJ, et al. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol 2009;53:483-90. [Crossref] [PubMed]
- Metra M, Ponikowski P, Dickstein K, et al. Advanced chronic heart failure: A position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2007;9:684-94. [Crossref] [PubMed]
- COOLEY DA. COLLINS HA, MORRIS GC Jr, CHAPMAN DW. Ventricular aneurysm after myocardial infarction; surgical excision with use of temporary cardiopulmonary bypass. J Am Med Assoc 1958;167:557-60. [Crossref] [PubMed]
- Athanasuleas CL, Buckberg GD, Stanley AW, et al. Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilation. J Am Coll Cardiol 2004;44:1439-45. [Crossref] [PubMed]
- Dor V, Civaia F, Alexandrescu C, et al. Favorable effects of left ventricular reconstruction in patients excluded from the Surgical Treatments for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg 2011;141:905-16, 916.e1-4.
- Jones RH, Velazquez EJ, Michler RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med 2009;360:1705-17. [Crossref] [PubMed]
- Dor V, Di Donato M, Sabatier M, et al. Left ventricular reconstruction by endoventricular circular patch plasty repair: a 17-year experience. Semin Thorac Cardiovasc Surg 2001;13:435-47. [Crossref] [PubMed]
- Di Donato M, Sabatier M, Dor V, et al. Effects of the Dor procedure on left ventricular dimension and shape and geometric correlates of mitral regurgitation one year after surgery. J Thorac Cardiovasc Surg 2001;121:91-6. [Crossref] [PubMed]
- Michler RE, Rouleau JL, Al-Khalidi HR, et al. Insights from the STICH trial: change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg 2013;146:1139-45.e6. [Crossref] [PubMed]
- Wechsler AS, Sadowski J, Kapelak B, et al. Durability of epicardial ventricular restoration without ventriculotomy. Eur J Cardiothorac Surg 2013;44:e189-92. [Crossref] [PubMed]
- Schmitz C, Biffi M, Sievert H, et al. Clinical benefits of less-invasive, device–based Left Ventricular Reconstruction: A hybrid option for patients with ischemic cardiomyopathy. J Am Coll Cardiol 2018;68:B330. [Crossref]
- Loforte A, Alfonsi J, Gliozzi G, et al. Less invasive ventricular enhancement (LIVE) as potential therapy for ischaemic cardiomyopathy end-stage heart failure. J Thorac Dis 2019;11:S921-8. [Crossref] [PubMed]
- Klein P, Anker SD, Wechsler A, et al. Less invasive ventricular reconstruction for ischaemic heart failure. Eur J Heart Fail 2019;21:1638-50. [Crossref] [PubMed]
- Clinical data. Available online: http://bioventrix.com/index. php/en/main. Online access, November 10th, 2018.
- Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231-48. Erratum in: J Am Coll Cardiol. 2015 May 26;65(20):2265. [Crossref] [PubMed]
- Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral valve repair in patients with heart failure. N Engl J Med 2018;379:2307-18. [Crossref] [PubMed]
- Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med 2018;379:2297-306. [Crossref] [PubMed]


