
Peri-operative right ventricular dysfunction—the anesthesiologist’s view
Introduction
Recent years have seen an increasing number of adult and pediatric patients with right ventricular dysfunction (RVD) presenting for both cardiac and non-cardiac surgery.
Right ventricular dysfunction is broadly defined as abnormal RV structure or function (1,2). Inclusive in this definition is consideration of the coupling between the RV and the pulmonary vascular bed. The etiologies of RVD are diverse with acute, acute-on-chronic and chronic subsets and can be summarized as follows (2):
- RV pressure loading pathologies such as precapillary pulmonary hypertension (PH), moderate to severe RV outflow tract obstruction/pulmonary stenosis, acute lung injury/acute respiratory distress syndrome, massive pulmonary thromboembolism, postcapillary PH due to elevated left atrial pressure from left heart systolic or diastolic dysfunction, or valvular disease (mitral, aortic);
- RV volume loading caused by congenital heart disease lesions or valvular pathologies such as large left to right intracardiac shunts (usually pre-tricuspid shunts such as large atrial septal defect), Epstein’s anomaly [tricuspid regurgitation (TR)], and repaired tetralogy of Fallot with free pulmonary regurgitation;
- Impaired RV contractility associated with cardiomyopathies, ischemia, single ventricle physiology, left ventricular assist devices (LVAD) and post-cardiotomy states.
Independent of the underlying pathophysiology, RVD is associated with poor clinical outcomes (2) and there is increasing recognition that peri-operative management of patients with RVD is challenging. With expanded therapies for PH and congenital heart disease and advanced technologies for mechanical support of the failing left ventricle (LV), anesthesiologists are likely to encounter more patients with RVD. This article highlights strategies to recognize, risk stratify, prevent and treat peri-operative RVD.
Pre-operative assessment
Successful pre-operative assessment of RV function requires a multi-modal approach with trending of clinical symptoms and signs, hematological, hemodynamic, and imaging parameters (2).
Clinical profile
Right heart failure (RHF) as strictly defined is “ a clinical syndrome due to an alteration of structure and/or function of the right heart circulatory system that leads to sub-optimal delivery of blood flow (high or low) to the pulmonary circulation and/or elevated venous pressures at rest or with exercise” (3). Consequently, while RV systolic dysfunction is an important cause of RHF, RHF can be present in the absence of RV systolic dysfunction due to volume or pressure overload lesions or RV diastolic dysfunction.
Assessment of recent clinical changes is important in assessing disease progression. Symptoms and signs of early RHF such as fatigue and exercise intolerance may be non-specific and difficult to identify, especially in the pediatric population who may be unable to verbalize abnormal sensations or in the older adult with associated co-morbidities. Clinical manifestations of more advanced RHF (Table 1) include peripheral edema, elevated jugular venous pressure (JVP), RV parasternal heave, hepatomegaly, right sided S3 gallop, holosystolic murmur at the left lower sternal border, abdominal discomfort and bloating, ascites, pleural effusions, right under quadrant pain from liver capsule stretch and poor growth curves in children. Patients with severe RHF may appear malnourished, tachypneic and cyanotic and are frequently in a state of vasodilation, interstitial fluid leakage, systemic inflammation and fever in the absence of infection (2). Systemic venous congestion may be harder to recognize in children than adults because extracellular fluid may not accumulate in obvious places like ankles, JVP can be difficult to interpret and sudden weight gains from fluid overload may be attributed to normal somatic growth (4). End organ effects of chronic right heart dysfunction are related to elevated central venous pressures with or without reduced cardiac output and include cardiorenal syndrome and cardio-hepatic syndrome. Splanchnic venous congestion and abnormal lymph flow can cause interstitial edema, reduced gastro-intestinal absorption and malnutrition. Increased intra-abdominal pressure may also contribute to renal failure (2). Hemodynamic studies have shown that venous congestion is more common than reduced cardiac output in children and adults with end-stage heart failure listed for heart transplant, with a consistent relationship between elevated right atrial pressure (RAP) and renal failure (4). Pre-operative laboratory studies may show elevated blood urea or creatinine (Cr) or altered hepatic synthetic function such as elevated prothrombin time. In chronic RHF, transaminases may be normal or minimally elevated whereas in acute RHF they are commonly high. Markers of cholestasis such as elevated bilirubin, gamma-glutamyl transpeptidase and alkaline phosphatase are independently associated with mortality in patients with heart failure and hyperbilirubinemia is a risk factor for poor outcomes in patients with PH (2). Protein losing enteropathy (PLE) may be seen with decreased serum albumin and increased stool alpha 1 antitrypsin (2).

Full table
Biomarkers
Risk stratification may be enhanced by inclusion of plasma biomarkers as part of the pre-operative assessment. The biomarkers currently receiving attention are those associated with myocardial stretch (brain type natriuretic peptide), myocardial fibrosis (galectin-3), and myocardial injury (high sensitivity troponin T) (5). Expert consensus guidelines recommend measuring brain natriuretic peptide (BNP) or N-terminal pro-brain natriuretic peptide (NT-proBNP) for diagnosis and prognosis in left heart failure and galectin-3 for added risk stratification (6). Elevated pre-operative BNP has been shown to be a strong predictor of major adverse cardiac events after non-cardiac surgery (7,8). Glomerular filtration rate (GFR) must be considered in the interpretation of BNP and NT-proBNP since reduced GFR levels results in elevation of NT-proBNP out of proportion to elevation of BNP (9). Despite this, when appropriate cut-off points are utilized, baseline elevation of BNP in renal dysfunction still have high prognostic value for detection of LV failure. High sensitivity troponin T is minimally affected by a patient’s renal function and is thought to reflect the pathophysiology of heart failure on a longer timescale (5,9). Although the role for biomarkers has not yet been established in RHF, studies have shown that NT-proBNP may have prognostic value for RHF accompanying PH (10) and acute pulmonary embolism (11).
Non-invasive imaging
Transthoracic echocardiography (TTE) remains the first line non-invasive imaging tool for pre-operative assessment. Some useful parameters include (12):
- RV afterload such as pulmonary artery systolic pressure (PASP) using peak velocity of TR jet and simplified Bernoulli equation;
- RV systolic functional indices such as tricuspid annular plane systolic excursion (TAPSE), right ventricle fractional area change (RVFAC) and tricuspid annular systolic velocity by tissue doppler;
- Variables of ventricular interdependency such as analysis of septal curvature. LV eccentricity index is the ratio of LV vertical to horizontal diameter measured from a short axis view of the LV at the mid-papillary level (e.g., parasternal short axis). D shape LV and eccentricity index >1 at end diastole reflects RV volume overload whereas at end systole it reflects RV pressure overload;
- Deformation indices such as RV longitudinal strain and strain rate by speckle tracking technology.
Reference values for echo parameters exist for both adults and children (13). Limitations of TTE include suboptimal image acquisition due to the complex RV geometry and its retrosternal position. Functional parameters measured are dependent on preload and angle of insonation and significant intra-observer variability exists in their acquisition. That said, deformation indices are less load dependent and RV longitudinal strain is emerging in the adult population as an independent prognostic marker in PH (14), an independent predictor of RV failure for LVAD implants (15,16) and a predictor of the need for high post-operative vasoactive support (17).
Due to the limitations associated with TTE, multiparametric echocardiographic approaches to RV assessment are proving to be more useful than over-reliance on any single index (18). An example is TAPSE which reflects systolic RV longitudinal function but does not take into account radial systolic function of the RV or segmental RV contraction (12). Radial systolic function is believed to play an important part in RV contractility in PH and can be severely impaired in severe PH patients despite a normal measured TAPSE. Combining TAPSE with afterload parameters in children to obtain a TAPSE/PASP ratio has recently been shown to be superior to TAPSE alone in differentiating patients with different NYHA functional class (FC)/modified Ross scores (18).
Despite advances with echocardiography, including an increased use of 3D echocardiography for volumetric parameters, cardiovascular magnetic resonance imaging (cMRI) remains the gold standard for quantitative non-invasive measurement of RV volume, function, mass, blood flow and tissue characterization including children with congenital heart disease (2). In contrast to most adults, young children and those with psychomotor delay may be unable to tolerate a cMRI without sedation or general anesthesia which can limit the feasibility of this investigation in hemodynamically marginal children. A more in-depth review of non-invasive imaging is discussed elsewhere in this journal (19).
Clinical prediction scores for RVD
Right ventricular dysfunction negatively impacts post-operative outcomes after cardiac surgery both in adults (20,21) and congenital heart disease patients (22-24). Recent studies have also shown that RVD is independently associated with major adverse cardiac events and a longer hospital stay in patients undergoing non-cardiac surgery (25). However, current pre-operative risk assessment for non-cardiac surgery in adults still focus on left heart dysfunction and do not incorporate markers of RV function. Contemporary publications addressing risk assessment scores for the pediatric population undergoing non cardiac surgery (26-28) as yet have not incorporated indices of RV function. One of the obstacles to incorporating RV function into risk stratification scores is the complexity, heterogeneity and lack of a universal definition for RVD which makes it difficult to compare or validate risk scores.
The majority of data on clinical prediction scores for RVD in both adults and children comes from two patient cohorts: (I) RVD associated with PH and (II) RVD in LVAD supported patients.
RVD associated with PH
Risk stratification scores from global adult PH registries have identified six consistent high yield variables indicative of deteriorating RV function (29): WHO FC, 6-minute walk distance (6MWD), NT-proBNP/BNP plasma levels, cardiac index, RAP and mixed venous oxygen saturation (SvO2). However, the majority of adult scoring systems do not incorporate age, underlying cause of PAH, co-morbidities, echocardiography or cMRI.
Variables which identify high risk pediatric PH patients include, WHO FC III/IV, clinical evidence of RV failure, syncope, mean pulmonary arterial pressure (mPAP)/mean systemic arterial pressure (mSAP) >0.75, RV systolic dysfunction, RAP >15 mmHg, elevated NT-proBNP, TAPSE, age <2 years and complexity/increased duration of procedure/anesthesia (30-32). Although these variables may serve as a guide for the risk of adverse peri-operative events and RV failure none have been formally validated in the peri-operative arena (28). Additionally, the impact of congenital syndromes such as Alagille’s or Williams syndrome have yet to be accounted for.
The European Pediatric Pulmonary Vascular Disease Network (EPPVDN) has developed a novel pediatric PH risk score based on variables defining a low- and high-risk group (30). Prospective validation of this novel risk score has commenced in children treated with “off-label” PH-targeted medication already approved for use in adults with PH (33,34).
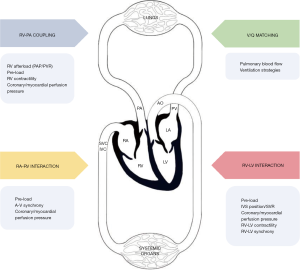
Experts have suggested that the degree of RV adaptation in PH may be a more accurate predictor of outcomes rather than isolated measurements of PA pressure in both the adult and pediatric PH population (23). RV adaptation in PH initially involves coupling of the RV to the high arterial load (Figure 1) and is maintained by increasing contractility and wall thickness followed by RV dilation and finally RV uncoupling (35). In PH when RV-PA coupling is still present, volumetric data such as right ventricular end diastolic volume (RVEDV), right ventricular end systolic volume (RVESV) and stroke volume (SV) may provide important prognostic information but needs further validation from large scale studies (35). Emerging studies have also recommended the ratio of TAPSE/PASP as a reflection of the extent of ventriculo-arterial uncoupling since TAPSE indicates RV contractile function and PASP a marker of RV afterload (2,18,36).
RVD in LVAD supported patients
Approximately 20–30% of patients (37,38) develop RVD after LVAD implantation. Currently no consistent identifiers can predict which patients will develop RVD.
Published literature addressing clinical prediction scores of RHF after LVAD implant includes parameters such as pre-implant age, vital signs, invasive hemodynamic metrics, echo parameters (e.g., TAPSE and TAPSE/RVEDV) mechanical ventilation, Cr and total bilirubin (16,37,38). RV longitudinal strain has been reported by several independent groups as a strong independent predictor of RV failure (15,16). However these scores only have modest performance in validation cohorts (16) and currently do not account for the heterogeneity of populations studied and dynamic intra-operative factors that increase pulmonary vascular resistance (PVR) and precipitate acute right heart dysfunction such as blood product transfusions, hypoxemia, acidosis, positive pressure ventilation, RV-LVAD interactions and change in RV volume loading and geometry with LVAD implantation (2,38). Recently, there has been interest in the use of the pulmonary artery pulsatility index (PAPi) as a predictor of RVD following LVAD placement. Pulmonary artery pulsatility index is defined as [(PA systolic pressure – PA diastolic pressure)/mean RAP]. A PAPi <1.85 has 94% sensitivity and 81% specificity for identifying RVD post-LVAD placement in adults (39,40). A cut-off point for this parameter in children has not been established, and PAPi may be “artificially high” when severe pulmonary regurgitation is present.
RV diastolic dysfunction
Only a few studies have assessed the importance of RV diastolic filling profiles in cardiac surgery (20). Although abnormal pre-operative RV diastolic profiles were associated with difficult separation from CPB (41), the independent value of RV diastolic function in cardiac surgery has not been clearly defined and may change depending on the underlying pathophysiology. In Tetralogy of Fallot, a restrictive RV filling profile is associated with low cardiac output state and longer ICU stay immediately post repair. However, in the long-term, restrictive RV diastolic function counteracts the effects of chronic pulmonary regurgitation and is associated with smaller RV, shorter QRS and increased exercise tolerance (20). Patients with single ventricle palliation are predisposed to diastolic ventricular dysfunction although the pathophysiology remains poorly understood and early detection is difficult (42). Diastolic and systolic dysfunction in the single ventricle physiology are thought to be closely intertwined (42). Clinical signs and symptoms of heart failure may manifest in a single ventricle palliation with progressive diastolic dysfunction even if contractility remains unchanged due to decreased stroke volume and a lower tolerance to increased end-diastolic pressures (42). A more in-depth review of RVD in congenital heart disease is found elsewhere in this journal (24).
Peri-operative management
The goals of successful peri-operative management include identifying patients at risk for RVD and employing risk mitigating strategies. Multidisciplinary discussions are vital in determining the risks and benefits of the procedure specific to the individual patient, determining optimal timing for non-urgent surgery, ensuring adequate pre-operative optimization, planning post-operative care and discussing feasible exit strategies should complications occur. Rescue strategies for decompensation include extra-corporeal membrane support, percutaneous RV assist devices and paracorporeal RV assist devices such Levitronix CentriMag (Abbott, Chicago, IL, USA).
Patients may be on a variety of medications depending on their underlying pathology, age and associated co-morbidities. Consideration should be given as to whether to continue these medications through the peri-operative period. Angiotensin-converting enzyme (ACE) inhibitors may cause profound vasodilatory hypotension in conjunction with anesthesia medications and it is advisable to withhold them 12–24 hours prior to surgery whereas chronic pulmonary vasodilator therapy such as sildenafil, endothelin receptor antagonists (ERA), and prostacyclin analogs should be maintained throughout the peri-operative period. Consideration should be given as to whether the patient’s clinical condition can be pre-optimized in the days or weeks prior to non-urgent procedures. Examples include pre-operative initiation of pulmonary vasodilators in untreated patients with PH, optimizing diuretic therapy or commencing inodilators such as milrinone or levosimendan in patients with significant venous congestion.
A pre-operative reduction in PVR or mPAP with inhaled pulmonary vasodilators indicates a reversible element to RV afterload and is a predictor for improved post-operative performance (43). Vasoreactivity testing for patients with isolated post-capillary PH may provoke pulmonary edema from increased LAP. However as post-capillary PH progresses, some patients develop small vessel disease and have mixed pre- and post-capillary PH and may also benefit from a pulmonary vasodilator (44).
There is no ideal anesthesia agent or technique and a variety of balanced multimodal regimes have been described in the literature (23). The physiologic goals, regardless of technique, are as follows:
- Decreasing RV afterload while maintaining RV preload, RV contractility and RV perfusion;
- Maintaining sinus rhythm, atrial contraction and atrio-ventricular (A-V) node synchrony;
- Maintaining LV output, interventricular septum (IVS) position and avoiding RV dilation;
- Avoiding the vicious cycle of systemic hypotension and RV ischemia.
Management of positive pressure ventilation in a patient with RVD is challenging and in some instances spontaneous ventilation can be maintained with the caveat that factors that may increase PVR such as hypoventilation, hypoxia, hypercarbia, atelectasis, airway obstruction and respiratory acidosis must be avoided. When positive pressure ventilation is initiated the goal is to prevent mechanical obstruction to pulmonary blood flow and a precipitous increase in RV afterload and reduction in preload during the inspiratory phase (45,46). Ventilatory parameters such as inspiratory: expiratory ratios (I:E time), tidal volume (PVR increases at high and low lung volumes), respiratory rate, and positive end-expiratory pressure (PEEP) should be optimized to generate the lowest possible mean airway pressure compatible with the desired lung recruitment, minute ventilation and gas exchange. The change from spontaneous ventilation to positive pressure ventilation is a critical time that requires vigilance and attention to detail. In patients previously known to be responsive to inhaled pulmonary vasodilators such as inhaled iloprost or nitric oxide, peri-intubation administration of these agents should be considered.
Adequate peri-operative monitoring is essential for early detection and treatment of RHF. Consideration should be given to placing a pre-induction arterial line for high risk patients and TTE during anesthesia induction can be useful to monitor RV dilation, IVS position and LV function especially during the transition from spontaneous to positive pressure ventilation. Since increases in central venous pressures may indicate RV failure, there should be a low threshold for placement of central venous catheters especially for longer or more complex procedures with anticipated fluid shifts and in selected cases pulmonary artery pressure catheters (PAC) can be useful.
Near infrared spectroscopy (NIRS) offers a non-invasive measurement of regional mixed arterio-venous oxygen saturation and is used for cerebral and somatic oximetry during the perioperative period. NIRS trends can be used as a surrogate marker of adequacy of blood flow to the brain and has been used to assess somatic tissue oxygen delivery when placed over the kidneys.
Focused transthoracic or transesophageal echocardiography is an important intra-operative diagnostic tool for any hemodynamically unstable patient (47). Echocardiographic indicators for peri-operative RV failure include signs of RV dilation (e.g., systolic or diastolic D shaping, moderate-severe TR), signs of impaired RV systolic function (e.g., TAPSE, RVFAC), and signs of elevated RV preload (e.g., plethoric IVC) (47). Acquisition of quantitative parameters can be time consuming and technically challenging in the operating room. Some experts have suggested that physicians should also be familiar with qualitative echocardiography assessment of RV dilation and function acknowledging that there may be a steep learning curve associated with acquiring this skill set (47).
Any systemic hypotension should be aggressively treated with judicious use of fluids and vasoactive medications to avoid the downward spiral of hypotension and RV ischemia. There is currently insufficient evidence to show superiority of one inotrope over another but first line agents to rapidly stabilize hemodynamics include epinephrine, dopamine and dobutamine. Some patients may benefit from initiation of vasoactive infusions prior to anesthesia induction (e.g., epinephrine or vasopressin). Inotropes such as epinephrine or dobutamine will increase RV contractility but may induce tachycardia which may impair diastolic filling and coronary perfusion. Vasopressor therapy such as norepinephrine, vasopressin, or terlipressin may be indicated to increase SVR, reduce leftward septal shift and improve tissue perfusion, including coronary/myocardial blood supply. Vasopressin has a theoretical advantage of being a selective systemic vasopressor and is not believed to increase PVR (48) as opposed to norepinephrine and epinephrine which can increase PVR at high doses. Inodilators such as milrinone or the calcium sensitizer levosimendan may be useful once hemodynamics have stabilized or as part of pre-optimization but are not first line therapy in the setting of systemic hypotension.
Tachyarrhythmias seen in association with RVD include sinus tachycardias, atrial fibrillation and atrial flutter. Cautious rate or rhythm control should be attempted, in addition to treatment of RV failure and its underlying cause (47). Bradyarrhythmias associated with RVD usually signify a pre-terminal event or severe ischemia of conduction system and should be treated immediately.
Post-operative management
After patients leave the operating room they remain at risk for adverse events and require vigilant monitoring of their oxygenation, ventilation and perfusion pressure. Appropriate monitoring of RV function and early detection of RV failure (including decreased forward flow and venous congestion) in the ICU remains challenging. PAC remains a useful modality in the adult population. Optimizing post-operative RV function involves balancing fluid titration with fluid restriction, optimizing heart rate and rhythm, maintaining normal acid-base status, avoiding positive pressure ventilation and hypoxia, adequate analgesia, temperature management and early use of pulmonary vasodilators and inotropes.
Conclusions
Peri-operative management of patients with significant RVD can be very challenging. Thorough pre-operative assessment, risk stratification and multi-disciplinary planning is essential for successful outcomes. Understanding the right heart as an integral part of the cardiopulmonary unit (Figure 1) with assessment of factors affecting inter-ventricular interactions, ventricular-arterial coupling, ventilation-perfusion matching and atrio-ventricular interactions should help the anesthesiologist formulate targeted peri-operative strategies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Martin Koestenberger, Harm-Jan Bogaard and Georg Hansmann) for the series “Right Ventricular Dysfunction” published in Cardiovascular Diagnosis and Therapy. The article was sent for external peer review organized by the Editor-in-Chief and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-426). The series “Right Ventricular Dysfunction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sanz J, Sánchez-Quintana D, Bossone E, et al. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:1463-82. [Crossref] [PubMed]
- Konstam MA, Kiernan MS, Bernstein D, et al. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation 2018;137:e578-622. [Crossref] [PubMed]
- Mehra MR, Park MH, Landzberg MJ, et al. Right heart failure: toward a common language. J Heart Lung Transplant 2014;33:123-6. [Crossref] [PubMed]
- Chen S, Dykes JC, McElhinney DB, et al. Haemodynamic profiles of children with end-stage heart failure. Eur Heart J 2017;38:2900-9. [Crossref] [PubMed]
- Dhir S, Dhir A. Cardiovascular risk assessment for noncardiac surgery: are we ready for biomarkers? J Cardiothorac Vasc Anesth 2020;34:1914-24. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/ACC guidelines for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:1810-52. [Crossref] [PubMed]
- Rodseth RN, Biccard BM, Manach Y, et al. The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide: a systematic review and individual patient data meta-analysis. J Am Coll Cardiol 2014;63:170-80. [Crossref] [PubMed]
- Zhang LJ, Li N, Li Y, et al. Cardiac biomarkers predicting MACE in patients undergoing noncardiac surgery: a meta-analysis. Front Physiol 2019;9:1923. [Crossref] [PubMed]
- Nishimura M, Brann A, Chang KW, et al. The confounding effects of non-cardiac pathologies on the interpretation of cardiac biomarkers. Curr Heart Fail Rep 2018;15:239-49. [Crossref] [PubMed]
- Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012;141:354-62. [Crossref] [PubMed]
- Coutance G, Cauderlier E, Ehtisham J, et al. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: a meta-analysis. Crit Care 2011;15:R103. [Crossref] [PubMed]
- Koestenberger M, Aptiz C, Abdul-Khaliq H, et al. Transthoracic echocardiography for the evaluation of children and adolescents with suspected or confirmed pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network. Heart 2016;102 Suppl 2:ii14-22. [Crossref] [PubMed]
- Koestenberger M, Nagel B, Ravekes W, et al. Reference values and calculation of z scores of echocardiographic measurements of the normal pediatric right ventricle. Am J Cardiol 2014;114:1590-8. [Crossref] [PubMed]
- Fine NM, Chen L, Bastiansen PM, et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging 2013;6:711-21. [Crossref] [PubMed]
- Grant AD, Smedira NG, Starling RC, et al. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol 2012;60:521-8. [Crossref] [PubMed]
- Kalogeropoulos AP, Kelkar A, Weinberger JF, et al. Validation of clinical scores for right ventricular failure prediction after implantation of continuous-flow left ventricular assist devices. J Heart Lung Transplant 2015;34:1595-603. [Crossref] [PubMed]
- Ting PC, Wu VC, Liao CC, et al. Preoperative right ventricular dysfunction indicates high vasoactive support needed after cardiac surgery. J Cardiothorac Vasc Anesth 2019;33:686-93. [Crossref] [PubMed]
- Koestenberger M, Avian A, Cantinotti M, et al. Tricuspid annular plane systolic excursion (TAPSE) in pediatric pulmonary hypertension: integrating right ventricular ejection efficiency (RVEe) into advanced multi-parametric imaging. Int J Cardiol 2019;274:296-8. [Crossref] [PubMed]
- Truong U, Meinel K, Haddad F, et al. Update on noninvasive imaging of right ventricle dysfunction in pulmonary hypertension. Cardiovasc Diagn Ther 2020;10:1604-24.
- Haddad F, Couture P, Tousignant C, et al. The right ventricle in cardiac surgery, a perioperative perspective: I. Anatomy, physiology, and assessment. Anesth Analg 2009;108:407-21. [Crossref] [PubMed]
- Bootsma IT, de Lange F, Koopmans M, et al. Right ventricular function after cardiac surgery is a strong independent predictor for long-term mortality. J Cardiothorac Vasc Anesth 2017;31:1656-62. [Crossref] [PubMed]
- Davlouros PA, Niwa K, Webb G, et al. The right ventricle in congenital heart disease. Heart 2006;92 Suppl 1:i27-38. [Crossref] [PubMed]
- Haddad F, Couture P, Tousignant C, et al. The right ventricle in cardiac surgery, a perioperative perspective: II. Pathophysiology, clinical importance, and management. Anesth Analg 2009;108:422-33. [Crossref] [PubMed]
- Santens B, Van De Bruaene A, De Meester P, et al. Diagnosis and treatment of right ventricular dysfunction in congenital heart disease. Cardiovasc Diagn Ther 2020;10:1625-45.
- Chou J, Ma M, Gylys M, et al. Preexisting right ventricular dysfunction is associated with higher postoperative cardiac complications and longer hospital stay in high-risk patients undergoing nonemergent major vascular surgery. J Cardiothorac Vasc Anesth 2019;33:1279-86. [Crossref] [PubMed]
- Faraoni D, Vo D, Nasr VG, et al. Development and validation of a risk stratification score for children with congenital heart disease undergoing noncardiac surgery. Anesth Analg 2016;123:824-30. [Crossref] [PubMed]
- Nasr VG, DiNardo JA, Faraoni D. Development of a pediatric risk assessment score to predict perioperative mortality in children undergoing noncardiac surgery. Anesth Analg 2017;124:1514-9. [Crossref] [PubMed]
- Brown ML, DiNardo JA, Nasr VG. Anesthesia in pediatric patients with congenital heart disease undergoing noncardiac surgery: defining the risk. J Cardiothorac Vasc Anesth 2020;34:470-8. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsedby AEPC, ESPR and ISHLT. J Heart Lung Transplant 2019;38:879-901. [Crossref] [PubMed]
- Bernier ML, Jacob AI, Collaco JM, et al. Perioperative events in children with pulmonary hypertension undergoing non-cardiac procedures. Pulm Circ 2018;8:2045893217738143. [Crossref] [PubMed]
- O’Byrne ML, Glatz AC, Hanna BD, et al. Predictors of catastrophic adverse outcomes in children with pulmonary hypertension undergoing cardiac catheterization: a multi-institutional analysis from the pediatric health information systems database. J Am Coll Cardiol 2015;66:1261-9. [Crossref] [PubMed]
- Schweintzger S, Koestenberger M, Schlagenhauf A, et al. Safety and efficacy of the endothelin receptor antagonist macitentan in pediatric pulmonary hypertension. Cardiovasc Diagn Ther 2020;10:1675-85.
- Hansmann G, Meinel K, Bukova M, et al. Selexipag for the treatment of children with pulmonary arterial hypertension: first multicenter experience in drug safety and efficacy. J Heart Lung Transplant 2020;39:695-706. [Crossref] [PubMed]
- Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol 2017;69:236-43. [Crossref] [PubMed]
- Guazzi M, Bandera F, Pelissero GP, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 2013;305:H1373-81. [Crossref] [PubMed]
- Simpson KE, Kirklin JK, Cantor RS, et al. Right heart failure with left ventricular assist device implantation in children: an analysis of the Pedimacs registry database. J Heart Lung Transplant 2020;39:231-40. [Crossref] [PubMed]
- Turner KR. Right ventricular failure after left ventricular assist device placement-the beginning of the end or just another challenge? J Cardiothorac Vasc Anesth 2019;33:1105-21. [Crossref] [PubMed]
- Morine KJ, Kiernan MS, Pham DT, et al. Pulmonary artery pulsatility index is associated with right ventricular failure after left ventricular assist device surgery. J Card Fail 2016;22:110-6. [Crossref] [PubMed]
- Alfirevic A, Sale S, Soltesz E. Foretelling right ventricular failure after left ventricular assist device implantation: the tale of the pulmonary artery pulsatility index. Anesth Analg 2019;128:8-10. [Crossref] [PubMed]
- Denault AY, Tardif JC, Mazer CD, et al. Difficult and complex separation from cardiopulmonary bypass in high-risk cardiac surgical patients: a multicenter study. J Cardiothorac Vasc Anesth 2012;26:608-16. [Crossref] [PubMed]
- Budts W, Ravekes WJ, Danford DA, et al. Diastolic heart failure in patients with the fontan circulation: a review. JAMA Cardiol 2020;5:590-7. [Crossref] [PubMed]
- Huang ST, Xu N, Sun KP, et al. The effect of perioperative administration of Treprostinil in infants with non-restrictive ventricular septal defect and severe pulmonary arterial hypertension. Pediatr Cardiol 2020;41:1334-9. [Crossref] [PubMed]
- Sokoliuk V, DiNardo JA, Brown ML. Never say never: the use of nitric oxide in patients with obstructed pulmonary veins: a case report. A A Pract 2019;12:205-7. [Crossref] [PubMed]
- Magder S, Guerard B. Heart-lung interactions and pulmonary buffering: lessons from a computational modeling study. Respir Physiol Neurobiol 2012;182:60-70. [Crossref] [PubMed]
- Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology 2005;103:419-28. [Crossref] [PubMed]
- Wanner PM, Filipovic M. The right ventricle-you may forget it, but it will not forget you. J Clin Med 2020;9:432. [Crossref] [PubMed]
- Siehr SL, Feinstein JA, Yang W, et al. Hemodynamic effects of phenylephrine, vasopressin, and epinephrine in children with pulmonary hypertension: a pilot study. Pediatr Crit Care Med 2016;17:428-37. [Crossref] [PubMed]


