Life span of patients with Eisenmenger syndrome is not superior to that of patients with other causes of pulmonary hypertension
Introduction
Pulmonary hypertension (PH) carries heterogeneous prognosis due to heterogeneous mechanisms. Congenital heart diseases can be associated with pulmonary arterial hypertension (PAH) through different conditions. Eisenmenger syndrome (ES) is one of the conditions in which a congenital heart disease that initially causes left-to-right shunt, induces an increase in pulmonary vascular resistance which eventually results in reversal of the direction of the shunt and development of cyanosis (1). There is an emerging percentage of adult patients with repaired congenital heart disease (2) developing PAH despite the closure of the shunt: the closed-shunt associated with PAH group. In the recently-published adult congenital heart disease Dutch registry, the overall prevalence of PAH in septal defects was 6% (3). In this group, PAH was related to ES in only 58% of the cases. Among the 42% remaining patients, some of them had PAH occurring despite closure of the heart defect.
Classically, patients with ES are considered to carry a better prognosis than patients with other causes of PH, closed-shunt associated with PAH, and patients with idiopathic PAH (IPAH) (4-10). All these studies focused on survival from diagnosis. Such comparisons are potentially biased because they are performed from the time of diagnosis rather than from the onset of the disease. The time interval between the beginning of the disease and the onset of symptoms cannot be precisely known, especially in cases with IPAH that may feature an extensive pre-clinical period of symptoms. Conversely, ES is associated with clinical signs and symptoms related to congenital heart disease. Therefore, the diagnosis of ES might be established closer from the beginning of the disease than for IPAH. Studying survival from diagnosis does not reflect the evolution of the disease entirely. We studied life span to add another dimension to the prognosis.
We aimed to determine if this better survival from diagnosis in ES patients was combined with a better overall life span compared to other causes of PH.
Our aims were (I) to determine survival from diagnosis as well as the life span in ES and closed-shunt associated with PAH in the current era, and (II) to compare survival from diagnosis and life span of ES and closed-shunt associated with PAH to other causes of PH.
Methods
Patients
Adult patients admitted to and/or who attended the regional referral centre for PH and congenital heart disease in La Timone Hospital, Marseille, France, were included in our specific database from January 2001 to September 2010. According to national health policy, patients older than 15 years and 3 months in France are usually referred to adult departments. PH was defined as an invasively-measured mean pulmonary artery pressure ≥25 mmHg (11). Patients in groups 2 and 3 according to the updated classification of PH, Nice 2013 (12) were not included in our study.
Patients in group 2 were excluded (n=2) because of the difference in management (first intention therapy consists in treating the left-sided heart disease). Patients in group 3 (with lung disease and/or hypoxemia) were not included (n=22) because they were managed in the respiratory disease department in another centre.
Patients who underwent heart-lung or lung transplantation or were lost to follow-up were censored at the time of the operation or at the time of the last personal contact with our centre.
Diagnosis of ES was based upon the presence of PAH at a systemic level and high pulmonary vascular resistance with reversed or bidirectional shunts through non-restrictive intracardiac or extracardiac communications (3,7,8,10). Patients with closed-shunt associated with PAH were defined as patients with PAH and previous surgical closure of a left-to-right shunt, without residual shunt at echocardiography and/or cardiac catheterization. Congenital heart disease classification was performed by three cardiologists specialising in congenital heart disease (Béatrice Bonello, Alain Fraisse, and Gilbert Habib) according to previous published classifications (12,13). The underlying defect was considered simple for atrial or ventricular septal defect, aorto-pulmonary window, persistent ductus arteriosus, and total or partial unobstructed anomalous pulmonary venous return. Complex defects included all other cases (combination of above-mentioned lesions, atrioventricular septal defect, and single ventricle…).
Data collection and entry
Data was retrospectively collected and derived from patients’ medical records. Demographic data collected was: date of birth, gender, date of diagnosis of PH confirmed by right heart catheterization, values of pulmonary artery pressure and vascular resistance, aetiology of the PH, type of congenital heart disease, and date of surgical closure of cardiac shunt if relevant. Follow-up was performed during scheduled outpatient clinics every 6 months, with clinical and echocardiography evaluation, or during hospitalizations. Outcome was defined as death, lung or heart and lung transplantation.
Data analysis
Statistical analysis was performed using SPSS 17.0. Categorical data was presented as counts (%) and continuous data as median (interquartile range). Mann-Whitney tests were used to compare age across groups. A Kruskal-Wallis test, followed by post-hoc Mann-Whitney tests was used to compare age across groups. Kaplan-Meier curves were used first to study event-free-survival from diagnosis to 15 years follow-up and secondly, to study survival according to the age in the three groups. Comparisons between groups were made using the log rank test. Patients who underwent lung or heart-lung transplantation were censored from further analysis at the time of transplantation because their subsequent survival would no longer reflect the survival pattern for PH, regardless of aetiology. P value <0.05 was considered as significant.
Results
Demographics
Out of 173 patients referred to our outpatients clinics for PH, 24 patients classified in group 2 (n=2) and in group 3 (n=22) were excluded.
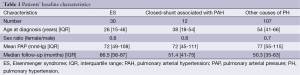
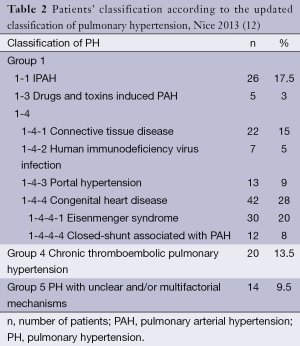
One hundred and forty nine patients were included and baseline characteristics are summarized in Table 1. Table 2 shows the repartition of patients according to the type of PH in accordance with the Nice classification (12). Thirty patients (20%) had ES, including three with Down syndrome. All of them had unrepaired congenital heart disease. Twelve patients (8%) had closed-shunt associated with PAH. Among the remaining 107 patients with other causes of PH, 26 (24%) had IPAH. There was no difference in mean pulmonary artery pressure between patients with other causes of PH compared to the patients with ES and closed-shunt associated with PAH (P=0.3 and P=0.1 respectively). Median age at diagnosis was lower in patients with ES and closed-shunt associated with PAH as compared to patients with other causes of PH (P<0.001 and P=0.008 respectively). Median age at diagnosis of patients with IPAH was 50 (range, 35-61) years. This was higher than that of ES (P<0.001).
Full table
Full table
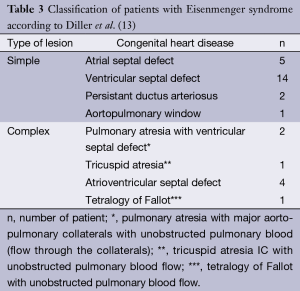
In patients with ES, 22 had simple cardiac lesion and 8 had complex cardiac lesion (Table 3). Twenty five patients had a post-tricuspid shunt and five had a pre-tricuspid shunt.
Full table
Outcomes (Table 4)

Full table
The median follow-up was 4.25 (range, 2.1-6.75) years. There was no difference in length of follow-up between patients with ES and closed shunt associated with PAH as compared to patients with other causes of PH (P=0.12 and P=0.2 respectively). Ten patients were lost to follow-up. Events during the first 15 years of follow-up occurred in five patients (16.6%) with ES, two patients (16.6%) with closed-shunt associated with PAH and 41 patients (38%) with other causes of PH. In the ES group, three patients died from complications of cyanosis (one from cerebral abscess and two from hemoptysis), one from heart failure, and one patient underwent heart-lung transplantation. In patients with closed-shunt associated with PAH, one patient died from heart failure and one patient experienced sudden cardiac death. In patients with other causes of PH, 28 patients (26%) died from complications of PH (heart failure and arrhythmias), 8 (7%) died from complication of their primary cause of PH: cirrhosis (n=4) and hemopathy (n=4); 3 (3%) died from infection and 2 (2%) underwent heart-lung transplantation. In patients with IPAH, 5 (20%) died from heart failure.
Survival from diagnosis
Survival from diagnosis was higher in patients with ES compared to patients with other causes of PH (logrank; P=0.02, Figure 1) or IPAH (logrank; P=0.04, Figure 2). Survival rates at 3, 6 and 9 years from diagnosis were respectively 73%, 50% and 47% for ES; 75%, 25% and 0% for closed-shunt associated with PAH; 65%, 23% and 9% for other causes of PH; and 58%, 35% and 15% for the IPAH subgroup.
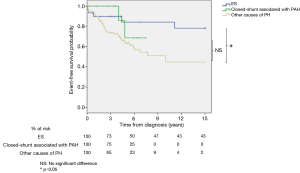
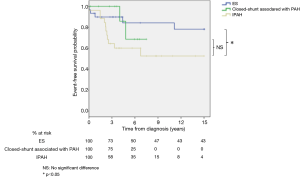
There was no difference for survival in patients with ES between simple versus complex lesion (logrank; P=0.8) and pre versus post-tricuspid shunt (logrank; P=0.3).
No difference in survival from diagnosis was observed between patients with a closed-shunt associated with PAH and patients with other causes of PH (logrank; P=0.3, Figure 1) or IPAH (logrank; P=0.2, Figure 2).
Life span
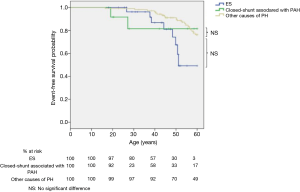
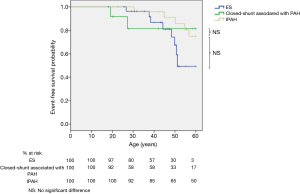
Survival according to the age was similar in patients with ES compared to patients with other causes of PH (logrank; P=0.2, Figure 3) or patients with IPAH (logrank; P=0.8, Figure 4). At 30, 40, 50 and 60 years, survival was respectively 96%, 87%, 67% and 49% for ES; whereas it was respectively 99%, 96%, 91%, and 76% for other causes of PH. Life span was similar between patients with closed-shunt associated with PAH and patients with other causes of PH (logrank; P=0.7, Figure 3) or patients with IPAH (logrank; P=0.7, Figure 4).
Discussion
To our knowledge, this is the first study comparing survival from diagnosis and life span in patients with ES, closed-shunt associated with PAH and other causes of PH. Life span was similar in the three groups of patients, despite a longer survival from diagnosis in patients with ES when compared to patients with other causes of PH and patients with IPAH.
Patients with PH carry an important heterogeneity with variable prognosis (4,5,13-22). To match previous studies (5,10,23-25), we performed our comparison with the subgroup of patients with IPAH. We found a slightly higher survival rate in our population of ES patients and IPAH patients, as compared to previous reports (10,23,24,26,27). These better outcomes may reflect the recent improvement in the management of those diseases with advanced therapies (14,28,29).
Morbidity and mortality in ES
In line with previous reports, we found that patients with ES carry a better prognosis from diagnosis than patients with IPAH (4-13,23,24,26). In ES right ventricular function remains preserved due to the persistence of a “fetal” heart physiology (9).
The fetal circulation is characterized by a dominant right ventricle with equal left and right ventricle pressure. In normal condition, at birth, right ventricular pressure drops and right ventricular wall thickness decreases. In ES, due to the left to right shunt, the regression of right ventricular wall thickness and the decrease of right ventricular pressure do not occur. In essence, fetal morphology persists throughout adulthood. In contrast, for adults who have severe PH due to other causes the markedly increased right ventricular afterload results in dilation and dysfunction of the right ventricle and right-sided cardiac failure.
How can we explain the lack of difference in life span between adult patients with ES and IPAH then? First, as we found, the diagnosis of ES is usually made at a younger age due to underlying symptoms of congenital heart disease; hence, they possibly live longer with the disease from the time of diagnosis. Conversely, IPAH may feature an extensive pre-clinical period of symptoms, whereas ES is associated with clinical signs and symptoms related to congenital heart disease. Secondly, in our ES populations, three of the four reported deaths resulted from well-defined complications of cyanosis (7,8,30). Cyanosis is responsible of abnormalities in platelets and coagulation pathways increasing the risk of both bleeding and thrombosis (31). Finally, there is a potential management bias when comparing patients with ES and other causes of PAH. Advanced therapies have been introduced in patients with IPAH following the recommendations from 2002 (25,32,33) with improvement in survival, whereas advanced therapies in ES have been recommended from 2006 (14,28,29). Comparison between survival in ES and IPAH should be performed after a longer period of uniform medical management.
Outcome of patients with closed-shunt associated with PAH
Modern management with early closure of the shunt, before the pulmonary vascular resistance increase, might decrease the incidence of PAH. However, some patients will develop PAH despite an early intervention. As previously reported patients with a closed-shunt associated with PAH carry a similar prognosis to patients with IPAH because they are prone to heart failure and sudden cardiac death (8,13). The median age of diagnosis is usually after the third decade (8,13). This should point the need for long term follow-up of patients after closure of a heart defect.
Study limitations
This is a retrospective study with a selection bias. First, we excluded patients lost to follow-up, and secondly we selected survivors referred to a tertiary PAH centre. Recent study suggests that survival of children with ES appears to be less than reported in adults (34,35). Therefore we can only make our observation on Eisenmenger patients who have reached adulthood. A population-based study including both paediatric and adult populations would be preferable.
Comparing each group of patients with PH according to the nice classification with one another would be interesting; our sample size did not allow such study. Multi-institutional studies should be performed.
Due to small number of patients in the closed-shunt associated with PAH group, our statistical analysis can have a lack of power. This could explain why, despite previous studies, we were unable to find any difference in survival with the ES group.
Amongst our patients with congenital heart disease and PAH, we did not have patients from groups 2 and 3 according to the Nice classification possibly because it is a very rare condition, or possibly because it is a more severe condition that does not allow survival through to adulthood. A larger group study may be necessary.
Amongst the patients with ES, there are different conditions: pre- versus post-tricuspid shunt with different physiopathology and evolution, both of which merit separate analysis. Unfortunately the small size of our group did not allow us to make different sub-groups for analysis. Our results and conclusions concern then mainly ES with a post-tricuspid shunt.
Conclusions
Although survival from the time of diagnosis differs between patients with ES, closed-shunt associated with PAH, other causes of PH as well as IPAH, their overall life span is similar. Long-term complications of cyanosis may counterbalance the more favourable hemodynamic profile of patients with ES.
Further multi-institutional studies with comparative management are required.
Acknowledgements
Disclosure: We thank Actelion Pharmaceuticals France for having taken care of the author fees for the Annual European Society of Cardiology Congress 2010, where this study was selected for abstract presentation. We thank Matthew Edward Jeremy Hall for his kind help in editing the text.
References
- Wood P. The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. Br Med J 1958;2:755-62. [PubMed]
- Marelli AJ, Mackie AS, Ionescu-Ittu R, et al. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 2007;115:163-72. [PubMed]
- Duffels MG, Engelfriet PM, Berger RM, et al. Pulmonary arterial hypertension in congenital heart disease: an epidemiologic perspective from a Dutch registry. Int J Cardiol 2007;120:198-204. [PubMed]
- Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023-30. [PubMed]
- Peacock AJ, Murphy NF, McMurray JJ, et al. An epidemiological study of pulmonary arterial hypertension. Eur Respir J 2007;30:104-9. [PubMed]
- McLaughlin VV, Presberg KW, Doyle RL, et al. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 2004;126:78S-92S. [PubMed]
- Daliento L, Somerville J, Presbitero P, et al. Eisenmenger syndrome. Factors relating to deterioration and death. Eur Heart J 1998;19:1845-55. [PubMed]
- Engelfriet PM, Duffels MG, Möller T, et al. Pulmonary arterial hypertension in adults born with a heart septal defect: the Euro Heart Survey on adult congenital heart disease. Heart 2007;93:682-7. [PubMed]
- Hopkins WE, Waggoner AD. Severe pulmonary hypertension without right ventricular failure: the unique hearts of patients with Eisenmenger syndrome. Am J Cardiol 2002;89:34-8. [PubMed]
- Hopkins WE, Ochoa LL, Richardson GW, et al. Comparison of the hemodynamics and survival of adults with severe primary pulmonary hypertension or Eisenmenger syndrome. J Heart Lung Transplant 1996;15:100-5. [PubMed]
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493-537. [PubMed]
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34-41. [PubMed]
- Diller GP, Gatzoulis MA. Pulmonary vascular disease in adults with congenital heart disease. Circulation 2007;115:1039-50. [PubMed]
- Tay EL, Papaphylactou M, Diller GP, et al. Quality of life and functional capacity can be improved in patients with Eisenmenger syndrome with oral sildenafil therapy. Int J Cardiol 2011;149:372-6. [PubMed]
- Haworth SG, Hislop AA. Treatment and survival in children with pulmonary arterial hypertension: the UK Pulmonary Hypertension Service for Children 2001-2006. Heart 2009;95:312-7. [PubMed]
- Souza R, Humbert M, Sztrymf B, et al. Pulmonary arterial hypertension associated with fenfluramine exposure: report of 109 cases. Eur Respir J 2008;31:343-8. [PubMed]
- Machado RD, Eickelberg O, Elliott CG, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S32-42. [PubMed]
- Kawut SM, Taichman DB, Archer-Chicko CL, et al. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest 2003;123:344-50. [PubMed]
- Kuhn KP, Byrne DW, Arbogast PG, et al. Outcome in 91 consecutive patients with pulmonary arterial hypertension receiving epoprostenol. Am J Respir Crit Care Med 2003;167:580-6. [PubMed]
- Le Pavec J, Souza R, Herve P, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med 2008;178:637-43. [PubMed]
- Opravil M, Pechère M, Speich R, et al. HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med 1997;155:990-5. [PubMed]
- Beghetti M. Congenital heart disease and pulmonary hypertension. Rev Port Cardiol 2004;23:273-81. [PubMed]
- Haworth SG, Hislop AA. Treatment and survival in children with pulmonary arterial hypertension: the UK Pulmonary Hypertension Service for Children 2001-2006. Heart 2009;95:312-7. [PubMed]
- Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156-63. [PubMed]
- Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012;142:448-56. [PubMed]
- D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343-9. [PubMed]
- Barst RJ, Ivy DD, Foreman AJ, et al. Four- and seven-year outcomes of patients with congenital heart disease-associated pulmonary arterial hypertension (from the REVEAL Registry). Am J Cardiol 2014;113:147-55. [PubMed]
- Gatzoulis MA, Beghetti M, Galiè N, et al. Longer-term bosentan therapy improves functional capacity in Eisenmenger syndrome: results of the BREATHE-5 open-label extension study. Int J Cardiol 2008;127:27-32. [PubMed]
- Galiè N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006;114:48-54. [PubMed]
- Diller GP, Dimopoulos K, Broberg CS, et al. Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J 2006;27:1737-42. [PubMed]
- Wedemeyer AL, Edson JR, Krivit W. Coagulation in cyanotic congenital heart disease. Am J Dis Child 1972;124:656-60. [PubMed]
- Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002;346:896-903. [PubMed]
- Humbert M, Barst RJ, Robbins IM, et al. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J 2004;24:353-9. [PubMed]
- Fraisse A, Jais X, Schleich JM, et al. Characteristics and prospective 2-year follow-up of children with pulmonary arterial hypertension in France. Arch Cardiovasc Dis 2010;103:66-74. [PubMed]
- van Loon RL, Roofthooft MT, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation 2011;124:1755-64. [PubMed]


