Lipoprotein-associated phospholipase A2 and carotid intima-media thickness in individuals classified as low-risk according to Framingham
Introduction
Coronary heart disease (CHD) affects over 16 million Americans, and is responsible for approximately 1 of every 6 deaths in the United States (1). Many patients with prognostically significant CHD are asymptomatic (2). Data from the Framingham Heart Study suggests that 64% of women, and 50% of men who died suddenly from CHD, had no prior symptoms suggesting the presence of CHD (1). Consequently, various methods of screening have been developed to facilitate early detection and intervention of those with subclinical CHD (3-5). The Framingham risk score (FRS) has long been used, and continues to be used, as a global estimator of CHD risk (6).
While the FRS does provide an estimate of CHD risk, it does not include certain known independent CHD risk factors, such as obesity or physical activity. Consequently, misclassification of CHD risk can occur. Data from the Third National Health and Nutrition Examination Survey (NHANES) show that a majority of men younger than 60 years old and women younger than 80 years old are at low 10-year predicted risk, according to FRS (7). Recent studies have suggested that in those classified as low risk by FRS, subclinical atherosclerosis may be still present (8-11).
Two emerging risk markers that have demonstrated effectiveness in predicting risk for future CHD events are carotid artery intima-media thickness (CIMT) and lipoprotein-associated phospholipase A2 (Lp-PLA2). It has been well established that CIMT is independently associated with CHD, and a consensus statement from the American Society of Echocardiography concludes that CIMT represents subclinical vascular disease, a marker of CHD (12-15). As a result, CIMT provides a useful surrogate end-point for CHD, and could be used to identify individuals at higher risk for CHD than those determined by more traditional risk factors alone (16,17).
Lp-PLA2 is a member of the phospholipase A2 family of enzymes and is produced by monocytes, macrophages, T lymphocytes, and mast and liver cells (18). Approximately two-thirds is found in the plasma bound to low density lipoprotein (LDL) cholesterol whereas the other one-third is bound to very-low-density lipoproteins and high density lipoprotein (HDL) cholesterol (19). Lp-PLA2 remains inactive until LDL undergoes oxidation within the arterial wall. Once LDLs are oxidized, Lp-PLA2 hydrolyzes the oxidized phospholipid creating two pro-inflammatory and pro-atherogenic constituents: lysophosphatidylcholine (LysoPC) and oxidized fatty acids (oxFA) (20). Lp-PLA2 has been shown to be predictive of future cardiovascular events (11,21).
Given the potential for FRS to misclassify individuals to a lower CHD risk, when there is a likelihood in many that more significant disease may be present, it is of great interest to develop improved risk models for identifying those at greatest risk for CHD, by including the addition of emerging risk markers. To date, no literature has examined the relationship between CIMT, Lp-PLA2, and more traditional risk factors in those currently classified as low risk by FRS. Therefore, the aims of this study were to determine if elevated CHD risk, as indicated by CIMT or Lp-PLA2 is present in those deemed low risk by FRS, and to explore the relationship between these two emerging risk markers.
Methods
Subjects
A total of 229 subjects (158 women and 71 men) were recruited. Prior to participation, subjects were informed of the procedures, risks, and benefits of the study and were required to sign an informed consent. All procedures were approved by the Ball State University Institutional Review Board. Inclusion criteria consisted of men and women aged 40-64 years and free of any known cardiovascular or metabolic disease and a 10-year CHD risk of <10%, as determined by FRS. FRS was calculated based on age, LDL and HDL cholesterol levels, blood pressure, smoking status, and diabetes (22).
Visit 1 procedures
Subjects were asked to report to the laboratory after a 12-hour fast. To control for plasma volume changes, subjects were seated for a minimum of 5 minutes prior to the blood draw. Prior to the blood draw, resting blood pressure was measured according to standardized procedures. Resting heart rate was taken by palpation of the radial artery for 30 seconds. Blood pressure, height, weight, waist and hip circumferences were measured according to standard procedures defined by the American College of Sports Medicine (23).
A 5 mL fasted sample of blood was collected. Approximately 0.5 mL of serum was alliquotted into each of 4 microcentrifuge tubes which were then frozen at –80 degrees Celsius for later batch analysis of Lp-PLA2. The remaining serum was analyzed for total cholesterol (TC), HDL cholesterol, triglycerides (TG), LDL cholesterol, and glucose concentration.
Subjects were then instructed on the proper use and procedures for PA assessment via the Actigraph GT1M accelerometer (Actigraph, Fort Walton Beach, FL, USA). Subjects were instructed to wear the accelerometer on their waist at the midline of the right thigh during all waking hours for 1 week.
Visit 2 procedures
CIMT was measured using the far wall of the right common carotid artery from longitudinal two-dimensional B-mode images using a Siemens ultrasound system (Sonoline Sienna, Japan) as previously described (24). Body composition was then measured via total body dual-energy X-ray absorptiometry (DXA) (GE Lunar Prodigy, enCORE 2007 version 11.40.004, GE Healthcare, Madison, WI, USA).
Lp-PLA2 analysis
Determination of Lp-PLA2 mass was assessed using an FDA approved ELISA assay (PLACTM test, diaDexus, Inc. San Francisco, CA, USA). This test is a sandwich enzyme immunoassay that uses two highly specific monoclonal antibodies (2C10 & 4B4) for the measurement of Lp-PLA2 concentration (25). The interassay coefficient of variation (CV) was between 0.1-6% for Lp-PLA2 mass, as reported by diaDexus.
Statistical analysis
Analyses were performed with IBM SPSS version 22.0 (Armonk, New York, USA). Independent sample t-tests were used to examine mean differences in study variables by gender and Lp-PLA2 mass risk classification. CIMT values ≥75th percentile for age were considered indicative of elevated CHD risk. Lp-PLA2 mass ≥200 ng/mL was considered elevated (11,15). Univariate Pearson correlations were used to determine relationships between Lp-PLA2 mass, CIMT, anthropometric, body composition, blood lipids, and physical activity variables. Significant variables determined by univariate analysis were selected for inclusion in hierarchical regression analysis with CIMT percentile and Lp-PLA2 mass entered as the dependent variables. Receiver operator curve (ROC) analysis was also conducted to examine the predictive ability of Lp-PLA2 on CIMT and CIMT on Lp-PLA2.
Results
Subject characteristics for all subjects and by gender are presented in Table 1. In the women, 19.5% of the subjects were considered at elevated risk according to the >75th percentile for CIMT (≥0.79 mm), and 34.6% were considered at elevated risk according to the >200 ng/dL cutpoint for Lp-PLA2 mass. In men, 4.6% were considered at elevated risk by CIMT (≥0.87 mm), and 73.8% by Lp-PLA2 mass.
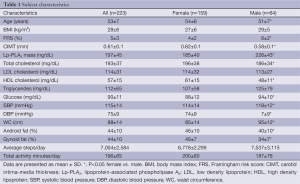
Full table
When subjects with elevated Lp-PLA2 mass (55 females, 47 males) were compared with those without, the subjects with high Lp-PLA2 mass demonstrated a significantly higher BMI, FRS percentile, LDL cholesterol, blood glucose, and WC (Table 2). They also had significantly decreased HDL cholesterol. Unexpectedly, those with elevated Lp-PLA2 mass demonstrated a significantly lower CIMT percentile as well.
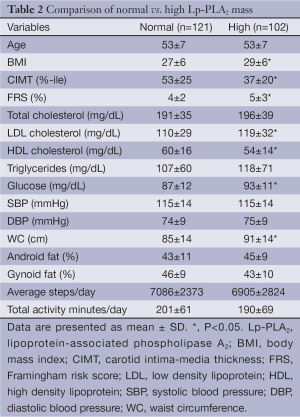
Full table
Univariate and multivariate predictors of CIMT and Lp-PLA2 mass
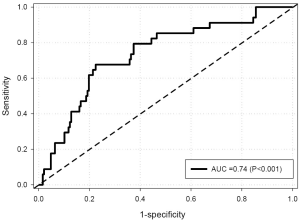
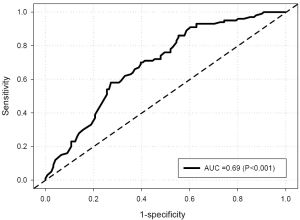
Univariate analysis (Table 3) revealed that CIMT was inversely related to Lp-PLA2 mass and age, and positively related to gender, and gynoid percent fat. FRS was not correlated to CIMT. Lp-PLA2 mass was significantly correlated with CIMT percentile, FRS, WC, gynoid percent fat, LDL and HDL cholesterol, and fasting blood glucose (BG). With Lp-PLA2 mass as the predictor for increased risk by CIMT (>75th percentile), ROC curve analysis resulted in an area under the curve (AUC) of 0.74 (P<0.001) for all subjects. At an Lp-PLA2 mass of 200 ng/mL, sensitivity and specificity were 0.85 and 0.51, respectively (Figure 1). When assessed by gender, AUC was 0.72 (P<0.001) and 0.47 (P=0.85) for females and males, respectively. With CIMT percentile as the predictor for increased Lp-PLA2 risk (≥200 ng/mL), AUC was 0.69 (P<0.001) for all subjects (Figure 2). At an FRS percentile of 75, sensitivity was 0.95 and specificity was 0.24. When assessed by gender, AUC was 0.72 (P<0.001) and 0.49 (P=0.93), for females and males, respectively.
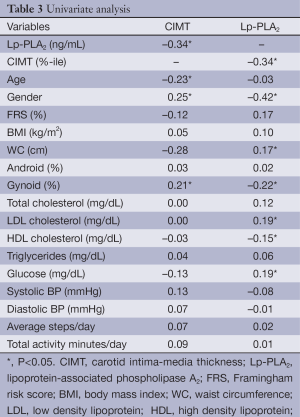
Full table
Hierarchical regression analysis was used to further assess the relationship of CIMT and Lp-PLA2 mass and more traditional risk markers (Table 4). After controlling for menopausal status, oral contraceptive use (OC), and hormone replacement therapy (HRT) (which explained 20% of the variance in CIMT), age (P=0.008) and Lp-PLA2 mass (P=0.002) were the significant independent predictors of CIMT percentile (R2=0.32, P<0.0001 for full model). When Lp-PLA2 mass was entered as the dependent variable, CIMT percentile (P=0.008), gynoid percent fat (P=0.003) and LDL cholesterol (P=0.005) were the significant independent predictors (R2=0.29, P<0.001 for full model), after controlling for menopausal status, OC, and HRT. Physical activity variables had no relationship to CIMT or Lp-PLA2 mass or activity.

Full table
Discussion
To our knowledge, the present study is one of the first to examine the relationship between the novel CHD risk markers of CIMT and Lp-PLA2 and individuals classified as low risk by FRS. Results indicate that even in those classified as low risk, a large proportion of our subjects were defined as higher risk according to Lp-PLA2 mass and activity criteria (11). In addition, those individuals detected with high Lp-PLA2 demonstrated a worsening of their overall risk profile compared to those who were not defined as high risk by Lp-PLA2 mass and activity criteria. We also found that in predicting CIMT percentile and Lp-PLA2 mass, CIMT did improve the prediction of Lp-PLA2 mass and vice versa. These findings appear to be more powerful with females than males. These findings support previous studies that found subclinical atherosclerosis present in those classified as low risk by Framingham, and may further show the potential for FRS to misclassify CHD risk (8-11).
What is unique in the current study is that in those “low risk” subjects, a significant proportion may already demonstrate an increased risk for CHD according to Lp-PLA2 standards. This may highlight the importance of emerging risk markers in identifying individuals at increased risk. The role of Lp-PLA2 is closely tied to vascular lesions and atheromas. Lp-PLA2 hydrolyzes oxidized LDL molecules within the arterial wall, which results in the attraction of monocytes. Therefore, Lp-PLA2 is directly involved in the formation of atherosclerosis and the production of vulnerable plaque that potentially leads to incident CHD (20). Our findings suggest that use of these novel, and not yet widely used risk markers, may aid in earlier detection of increased CHD risk, and lead to more timely intervention to potentially prevent incident CHD.
The current study does not have CHD outcome data on these subjects, as it was beyond the scope of the study. However, the Women’s Health Study (WHS), examining women age ≥45 years, reported a mean mass of 105±41 ng/dL in those that did not experience a CHD event (26). Other studies have reported higher mean Lp-PLA2 mass values in those without a CHD event, but included individuals with hypertension, dyslipidemia, diabetes mellitus, and current smokers, which is why it is not surprising that their Lp-PLA2 values are higher than the current study (27,28). Results from the current study appear to be most similar to that of the WHS, with the exception that we included men in the analysis. Men in our analysis had significantly higher Lp-PLA2 values, which may explain the higher values in our study vs. that of the WHS.
A few studies have examined the relationship between Lp-PLA2 and CIMT. Campo et al. examined the relationship between CIMT and Lp-PLA2 activity in 190 Sicilian middle-aged subjects and found no relationship between these measures (29). Subjects for this study, however, included those with diabetes, hypertension, dyslipidemia, as well as current smokers. In addition, Lp-PLA2 mass was not examined, which appears to demonstrate the more potent relationship in our current study. Kiortsis et al., examined the relationship between CIMT and Lp-PLA2 mass and activity in 100 subjects with known dyslipidemia and 67 matched controls without dyslipidemia (30). Like Campos et al., they found no relation between CIMT and Lp-PLA2. In addition to dyslipidemia, 15% of control subjects and 24% of subjects with dyslipidemia were current smokers (30). Results from the current study are in contrast to the studies by Campos and Kiortsis, in that CIMT and Lp-PLA2 mass were independent predictors of each other. All three studies were of similar age and BMI. It is possible that the addition of other CHD risk factors in the other studies may have impacted this relationship.
In contrast to the studies from Campos and Kiortsis, Ikonomidis et al. also examined the relationship between CIMT and Lp-PLA2 in 111 patients with documented CAD, and found that those with Lp-PLA2 values >234.5 ng/mL, CIMT was greater (31). Furthermore, they found, in agreement to the current study, that Lp-PLA2 was an independent predictor of CIMT. What is interesting when comparing our two studies, however, is that our study found an inverse relationship between CIMT and Lp-PLA2, whereas Ikonomidis found a positive relationship. Reasons for this are unclear, but may be due to the difference in the overall risk profiles of our cohorts.
Studies examining CIMT have rarely utilized the >75th percentile criteria in their analysis. Salonen et al. (32) found that in 1,200 Finnish men (a population with high incidence morbidity and mortality from CHD), 20% had elevated CIMT, defined as >1.0 mm between the intimal-luminal interface and the medial-adventitial interface in the common carotid below the carotid bulb (32). Direct comparison with our study is difficult, given the differing cohorts studied and definitions of elevated CIMT. The Rotterdam study, a prospective cohort study that examined over >7,000 men and women >55 years of age in several home for the elderly, reported 25% had elevated CIMT (33). In contrast, the current study found 4.6% of our men, and 19.5% of our women had elevated CIMT. The Rotterdam cohort was older than the current study. The arterial wall naturally thickens with age, regardless of the degree of atherosclerosis, so this is likely a factor in the greater prevalence numbers seen there (34).
One study that did utilize the 75th percentile criteria for elevated CIMT examined middle-aged (mean 47 years) firefighters (35). Mean FRS in their subjects was 3% (vs. 5% in the current study). In a sample size of 50 subjects, 27 (54%) were shown to have elevated CIMT. Despite the lower FRS in their study across all subjects compared to ours, subjects in this study were often on medications, were obese, and/or were pre-diabetic or diabetic. This likely explains the greater prevalence in high CIMT seen here.
A somewhat surprising finding of our study was that CIMT values were opposite of what was expected in that, high Lp-PLA2 mass and activity risk was associated with significantly lower CIMT percentile values. Reasons for this finding are unclear, but given the greater number of females vs. males in the current study, it could simply be a reflection of gender, as gender was significantly correlated to CIMT and Lp-PLA2 mass and activity.
The present study has limitations. First, the population studied was a self-referred, homogenous group aged 45-69 years, almost entirely Caucasian, which was beneficial in that it limits confounding variables within the group. Secondly, because this study was delimited to low risk individuals (FRS: 1-9%), range restriction may have decreased the significance of the contribution of the FRS in regression analysis.
Conclusions
In conclusion, results from the current study indicate that even in those classified as low risk by FRS, evidence of elevated CHD risk may exist, reflected by the emerging risk markers of CIMT and Lp-PLA2. Our data adds support that more traditional risk markers have limitations in their ability to discern those who may be at increased risk for CHD. The implementation of newer emerging risk markers may have significant utility in the earlier detection of CHD, particularly as cost and availability of these tests become more reasonable. The relationship of CIMT to Lp-PLA2 remains unclear; however we did show that CIMT and Lp-PLA2 mass were independently related, in contrast to previous research. Due to the similar endpoints of these two markers, additional research is needed to elucidate the relationship between these two markers of CHD risk.
Acknowledgements
Funding: This study was supported by diaDexus, Inc., South San Francisco, CA, USA.
Disclosure: The authors declare no conflict of interest.
References
- Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:399-410. [PubMed]
- Pasternak RC, Abrams J, Greenland P, et al. 34th Bethesda Conference: Task force #1--Identification of coronary heart disease risk: is there a detection gap? J Am Coll Cardiol 2003;41:1863-74. [PubMed]
- Greenland P, Smith SC Jr, Grundy SM. Improving coronary heart disease risk assessment in asymptomatic people: role of traditional risk factors and noninvasive cardiovascular tests. Circulation 2001;104:1863-7. [PubMed]
- Simon A, Chironi G, Levenson J. Performance of subclinical arterial disease detection as a screening test for coronary heart disease. Hypertension 2006;48:392-6. [PubMed]
- Liebson PR, Amsterdam EA. Prevention of coronary heart disease. Part II. Secondary prevention, detection of subclinical disease, and emerging risk factors. Dis Mon 2000;46:1-123. [PubMed]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143-421. [PubMed]
- Marma AK, Berry JD, Ning H, et al. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2006. Circ Cardiovasc Qual Outcomes 2010;3:8-14. [PubMed]
- Lakoski SG, Greenland P, Wong ND, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as "low risk" based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA). Arch Intern Med 2007;167:2437-42. [PubMed]
- Abe Y, Rundek T, Sciacca RR, et al. Ultrasound assessment of subclinical cardiovascular disease in a community-based multiethnic population and comparison to the Framingham score. Am J Cardiol 2006;98:1374-8. [PubMed]
- Michos ED, Nasir K, Braunstein JB, et al. Framingham risk equation underestimates subclinical atherosclerosis risk in asymptomatic women. Atherosclerosis 2006;184:201-6. [PubMed]
- Davidson MH, Corson MA, Alberts MJ, et al. Consensus panel recommendation for incorporating lipoprotein-associated phospholipase A2 testing into cardiovascular disease risk assessment guidelines. Am J Cardiol 2008;101:51F-57F. [PubMed]
- Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol 1997;146:483-94. [PubMed]
- Craven TE, Ryu JE, Espeland MA, et al. Evaluation of the associations between carotid artery atherosclerosis and coronary artery stenosis. A case-control study. Circulation 1990;82:1230-42. [PubMed]
- Rosvall M, Janzon L, Berglund G, et al. Incident coronary events and case fatality in relation to common carotid intima-media thickness. J Intern Med 2005;257:430-7. [PubMed]
- Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008;21:93-111. [PubMed]
- Roman MJ, Naqvi TZ, Gardin JM, et al. American society of echocardiography report. Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society for Vascular Medicine and Biology. Vasc Med 2006;11:201-11. [PubMed]
- Roman MJ, Naqvi TZ, Gardin JM, et al. Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr 2006;19:943-54. [PubMed]
- Koenig W, Twardella D, Brenner H, et al. Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol 2006;26:1586-93. [PubMed]
- Caslake MJ, Packard CJ, Suckling KE, et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis 2000;150:413-9. [PubMed]
- MacPhee CH, Moores KE, Boyd HF, et al. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J 1999;338:479-87. [PubMed]
- Grundy SM, Pasternak R, Greenland P, et al. AHA/ACC scientific statement: Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol 1999;34:1348-59. [PubMed]
- Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47. [PubMed]
- Pescatello LS, Arena R, Riebe D, et al. eds. ACSM's guidelines for exercise testing and prescription, 9th ed. Philadelphia: Lippincott Williams & Wilkins, 2014.
- Pignoli P, Tremoli E, Poli A, et al. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986;74:1399-406. [PubMed]
- Burke GL, Evans GW, Riley WA, et al. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke 1995;26:386-91. [PubMed]
- Blake GJ, Dada N, Fox JC, et al. A prospective evaluation of lipoprotein-associated phospholipase A(2) levels and the risk of future cardiovascular events in women. J Am Coll Cardiol 2001;38:1302-6. [PubMed]
- Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2004;109:837-42. [PubMed]
- Persson M, Nilsson JA, Nelson JJ, et al. The epidemiology of Lp-PLA(2): distribution and correlation with cardiovascular risk factors in a population-based cohort. Atherosclerosis 2007;190:388-96. [PubMed]
- Campo S, Sardo MA, Bitto A, et al. Platelet-activating factor acetylhydrolase is not associated with carotid intima-media thickness in hypercholesterolemic Sicilian individuals. Clin Chem 2004;50:2077-82. [PubMed]
- Kiortsis DN, Tsouli S, Lourida ES, et al. Lack of association between carotid intima-media thickness and PAF-acetylhydrolase mass and activity in patients with primary hyperlipidemia. Angiology 2005;56:451-8. [PubMed]
- Ikonomidis I, Kadoglou NN, Tritakis V, et al. Association of Lp-PLA2 with digital reactive hyperemia, coronary flow reserve, carotid atherosclerosis and arterial stiffness in coronary artery disease. Atherosclerosis 2014;234:34-41. [PubMed]
- Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb 1991;11:1245-9. [PubMed]
- Bots ML, Hoes AW, Koudstaal PJ, et al. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation 1997;96:1432-7. [PubMed]
- Nagai Y, Metter EJ, Earley CJ, et al. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation 1998;98:1504-9. [PubMed]
- Ratchford EV, Carson KA, Jones SR, et al. Usefulness of coronary and carotid imaging rather than traditional atherosclerotic risk factors to identify firefighters at increased risk for cardiovascular disease. Am J Cardiol 2014;113:1499-504. [PubMed]


