
Anticoagulation management in adult patients with congenital heart disease: a narrative review
Background
The number of adults with congenital heart disease (ACHD) is steadily increasing as a consequence of new treatment options and advances in medicine (1-3) as the number of newborns with CHD remains constant (4). ACHD patients currently account for about two-thirds of the patient population with CHD (5), Compared to about one-half3 at the beginning of the new millennium. Even severe CHD is becoming more prevalent in adults, as shown by data from Quebec (5,6). In this context further morbidity in these patients in regards of arrhythmias, stroke or pulmonary embolism increases as well (Figure 1).
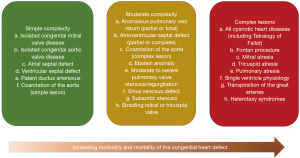
Arrhythmias, heart failure, pulmonary embolism and stroke are the most common reasons for unplanned hospital admission in patients with congenital heart disease. Patients with ACHD also have a markedly increased risk of stroke compared to the general population (7-10). ACHD patients aged of 55 years or younger had a stroke rate 9 to 12 times higher than in the general population, and 1 in 11 male and 1 in 15 female of the ACHD patients with ages from 18 to 64 years had a reported stroke (11,12). According to previous studies, the ischemic stroke rate is 28.4 per 100,000 person years with ACHD in comparison to a control population with a stroke rate of 2.6 per 100,000 person years (8). The same is true for reported haemorrhagic stroke with 1.18 per 10,000 person years in ACHD patients (7). Most commonly treatment is complicated by comorbidities like heart failure, arrhythmias, arterial hypertension or impaired organ function of liver or kidneys (13). Although prevention of stroke and pulmonary embolism has traditionally relied on vitamin K antagonists (VKA) such as warfarin, recent guidelines suggest the use of non-vitamin K oral anticoagulants (NOAC) in patients with ACHD presenting with atrial fibrillation (AF), stroke or pulmonary embolism (1,14). This is equally true for the highly prevalent arrhythmias described in the ACHD cohort with atrial flutter (AFl) and intraatrial tachycardias resulting from previous surgical procedures (1,14). In addition, patients with certain complex congenital heart disease are at high risk of embolic complications even without diagnosed arrhythmias. This includes patients with a univentricular heart in Fontan circulation, atrial switch operation for transposition of the great arteries, or ACHD with advanced heart failure and associated complications (15,16).
The CHA2DS2-VASc score, or an abbreviated version without sex as an additional factor are recommended for initial stroke risk estimation in patients with AF (1,14). Ease of use and wide-spread knowledge of the score underscore its usefulness, although the predictive ability is modest. More recently, biomarkers have been proposed (e.g., troponin, BNP, GDF-15, and others) to refine stroke risk prediction (17,18). Thus, there is still a gap of studies addressing this issue in ACHD patients, who are much younger than patients with AF and are likely to have a higher stroke risk. Unfortunately, risk of stroke and embolism can only be roughly estimated based on results of single-centre studies or data with expert consensus level from current guidelines for planning treatment. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-631).
Objectives
The objective of this review to summarize the different indications for oral anticoagulation (OAC) in adult patients with congenital heart disease. In addition the most recent study results were included and how these may contribute to a better algorithm in patient selection for a certain underlying congenital heart defect. Further a focus of the review is to give examples of special situations were OAC might be indicated for example implantation of a prosthetic heart valve or anticoagulation has to be carefully planned like during pregnancy.
Methods
Literature search
A literature search was performed in PubMed covering publications up to April 2020. The following combinations of keywords were used: anticoagulation and CHD, morbidity and mortality in ACHD, guidelines anticoagulation and ACHD, guidelines diagnostic and treatment of patients with congenital heart disease. These search terms had to be identified anywhere in the text in the articles. The authors as well did choose literature depicting the current guidelines or multicenter trials, however most of the literature are of observational studies and guidelines for patients with ACHD have the evidence level of expert opinion.
Both qualitative and quantitative studies were considered to elucidate the use of the different aspects regarding congenital heart disease, anticoagulation and combination of both. The search was restricted to original research, humans, and papers published in English at any date. All abstracts were reviewed to assess whether the article met the inclusion criteria. The key inclusion criterion was the presence of any cardiovascular disease in combination with pregnancy. Most of the suggestions depend on the summary statement of the American Heart Association (1) and the guidelines of the European Society of Cardiology (2,14). After this selection process, a manual search of the reference lists of all eligible articles was performed. Two authors (i.e., EZ and CS) assessed independently the methodological quality of the qualitative and quantitative studies prior to their inclusion in the review.
Stroke risk assessment using the CHA2DS2-VASc score in ACHD patients
It is important to address the issue of thromboembolic cerebrovascular disease as it is a major cause of morbidity in ACHD patients (7,8,19). Indeed the standardized incidence rates for ischemic stroke show a 9- to 12-fold increased risk for ACHD patients below 55 years of age with heart failure, diabetes and previous myocardial infarction as the strongest risk factors (14). Some anatomic defects have been identified which particularly increase the risk of ischemic stroke with uncorrected cyanotic heart disease, such as Eisenmenger physiology, native atrial septal defects and Fontan circulation (14), In approximately 25% of the ACHD patients the occurrence of stroke was associated with the loss of the sinus rhythm (14,20). As a result, patients with Fontan circulation, Eisenmenger or residual right-to-left shunt should in general receive OAC therapy (14,20). An important point to consider in patients with central cyanosis is the fact that these patients on the one hand have a higher risk of stroke, however on the other hand they have a higher risk of as well bleeding as the coagulation system is defective in these patients as well (14,21). As a result it is suggested that cyanotic patients should receive OAC therapy if they have an arrhythmia with most commonly AF (14,21). In two recent single- and multicenter studies (with a majority of patients with moderate or complex severity), treatment with a NOAC was not associated with an increased risk as long as the patients did not have an impaired renal function (22,23).
In lower risk patients, therapy schemes as recommended for other types of acquired or structural heart disease and the use of the CHA2DS2-VASc score as recommended by guidelines seems to be also appropriate in ACHD (1,24). The described cohort of patients with a low risk and AF with a CHA2DS2-VASc score of 0 in men and 1 in women are currently not recommended for OAC therapy (14,24). In general it is suggested that patients with moderate and complex ACHD—specifically patients with intracardiac repair, Fontan circulation, cyanosis and systemic right ventricle—and intra-atrial reentrant tachycardia (IART), AF or AFl should be anticoagulated even when the CHA2DS2-VASc score is 0 in men and 1 in women (1,21). The recommendations regarding anticoagulation in ACHD patients, special types of diseases and respective CHA2DS2-VASc score are shown in Table 1.
Table 1
| Underlying disease in ACHD patients | Arrhythmia | Anticoagulation | Target INR |
|---|---|---|---|
| CHA2DS2-VASc score ≥0 and cyanosis | Atrial fibrillation/atrial flutter/intra-atrial reentrant tachycardia | VKA | 2.0–3.0 |
| CHA2DS2-VASc score ≥0 and intracardiac repair | Atrial fibrillation/atrial flutter/intra-atrial reentrant tachycardia | VKA | 2.0–3.0 |
| CHA2DS2-VASc score ≥0 and Fontan surgery | Atrial fibrillation/atrial flutter/intra-atrial reentrant tachycardia | VKA | 2.0–3.0 |
| CHA2DS2-VASc score ≥0 and systemic right ventricle | Atrial fibrillation/atrial flutter/intra-atrial reentrant tachycardia | VKA | 2.0–3.0 |
| CHA2DS2-VASc score =0 | Atrial fibrillation/atrial flutter/intra-atrial reentrant tachycardia | No anticoagulation | – |
| CHA2DS2-VASc score ≥1 in men and ≥2 in women | Atrial fibrillation/atrial flutter/intra-atrial reentrant tachycardia | VKA or NOAC | 2.0–3.0 or no additional monitoring in case of NOAC |
ACHD, adults with congenital heart disease; VKA, vitamin K antagonists; NOAC, non-vitamin K oral anticoagulants.
The CHA2DS2-VASc score was defined to assess the risk of stroke in patients with non-valvular AF (20) and OAC therapy is normally recommended when the score is ≥1 in men and ≥2 in women as recommended by the current guidelines (14,24). The score includes several risk factors with congestive heart failure/LV dysfunction (C), hypertension (H), age ≥75 years (A), diabetes mellitus (D), previous stroke (S), vascular disease (V), age 65–74 years (A) and female sex category (Sc). Each risk factor gives a score of 1, except age ≥75 years and previous stroke, which are associated with a score of 2. Furthermore, female sex alone does not give a score of 1, unless the patient is 65 years or above (14,20,24). According to a recent position paper and a multicentre study NOAC therapy in ACHD should also be assessed according to the CHA2DS2-VASc score (14,26).
Risk scores predicting thromboembolic complications in patients with AF or AFl do not consider the presence, type, or severity of the congenital heart disease. Thus, in the absence of large-scale prospective studies, the guidelines recommend a similar approach to anticoagulation/ transoesophageal echocardiography prior to cardioversion or other diagnostic or therapeutic procedures. However, it should be considered that adults with moderate or complex forms of ACHD may be predisposed to thrombus formation even in the absence of atrial tachyarrhythmias and thus selected patients might benefit of an OAC therapy to prevent cerebrovascular events (1,14).
The use of novel oral anticoagulants (NOAC) in ACHD patients
Most commonly vitamin-K antagonists (VKA) are used in ACHD patients because of the lack of experience with NOACs in ACHD patients. This is due to the fact that VKA were traditionally used and there is still limited experience with NOACs. Therefore, the current guidelines most commonly endorse the use of VKA in ACHD patients (1,14). However, the current status regarding the use of NOACs has to be reassessed in light of new study results and publications specifically addressing this issue in ACHD patients (1,14,16,21-23,27-34). The current recommendations as covered by the guidelines summarized in Figure 2. The general results in the non-ACHD population indicate that NOACs had a favourable risk-benefit profile, with significant reductions in stroke, intracranial haemorrhage, and mortality, and with similar major bleeding as for VKA, but increased gastrointestinal bleeding risk (35). The major advantage of NOACs is that regular monitoring of anticoagulation level is not required since NOACs have a more predictable anticoagulant effect and have been demonstrated to be at least as efficient and safe as VKA (36,37). The major disadvantages often referred to when the use of NOACs is discussed in ACHD patients are unknown pharmacokinetic/pharmacodynamic effects. However, the effect of impaired renal or liver function as well as interactions with often prescribed medications have been published and should not—in isolation—be regarded as a contraindication in ACHD patients (36). Although the overall number of ACHD patients is increasing (5,38), randomized studies are still difficult to conduct in ACHD patients, mainly due to the smaller number of cases, the heterogeneity, even within the same CHD group, the low event rate and concomitant co-morbidities with possible effects on coagulation. Some observational studies have reported on the use of NOACs in ACHD. These include a larger single-centre study (23,27) and the worldwide NOTE registry (22). In both studies (22,23) the majority of ACHD patients was of moderate or severe complexity. Therefore, these reports address the knowledge gap regarding these patient cohorts including patients with Fontan circulation or arrhythmias in need of anticoagulation (1). The data of both studies suggest that in cohorts with predominantly moderate and complex forms of congenital heart disease, potentially including Fontan circulation, NOACs might be used safely. The use of NOACs in Fontan was assessed only in one additional study (16) of 21 Fontan patients as well reporting low numbers of bleeding events or thrombosis. However, there are some reports in the literature on clotting in Fontan Patients on NOACS (29,39,40). Moreover, randomized trials, long-term outcome studies and research into often associated pre-existing abnormalities in clotting and fibrinolysis (41,42) which make the use of NOAC more challenging are largely missing in such patients. Even in the largest study of patients with Fontan surgery, the NOTE registry (22), only 74 Fontan patients were included. Also to keep in mind, we have an overall young Fontan population in which we have to consider the role of contraception and pregnancy and anticoagulation management during those times. Therefore, we can not necessarily extrapolate data from the adults with structurally normal hearts to the Fontan population. At that time it would be too early to endorse the use of NOACs in the Fontan population.
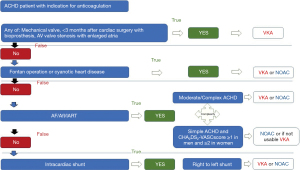
The overall message of both above mentioned observational (non-controlled) studies (19,20) are that NOACs were safe and effective in the short term for the indication to minimize thrombotic events in patients with predominantly moderate and complex forms of congenital heart disease in adults, including those with various forms of valve disease and as well bioprosthetic valves (22,23).
The predominant indication in ACHD patients remains arrhythmias with AF presenting the most common problem. The prevalence of AF is rapidly increasing in the aging population with ACHD, and is associated with considerable morbidity. In ACHD patients AF is already the leading presenting arrhythmia over the age of 50 (14,29,43). While NOACs can be prescribed in patients with simple defects (1) their use may also be considered in moderate or complex ACHD patients (22,23).
Special considerations regarding anticoagulation in ACHD patients
Prosthetic valves
Congenital heart disease patients might require valve replacement, especially in patients with congenital aortic stenosis, Tetralogy of Fallot, atrioventricular septal defect, mitral valve disease or Ebstein’s disease (2,21). Before valve replacement, the type of heart valve which determines the anticoagulation regime has to be discussed thoroughly with the patient. In the case of a mechanical heart valve regardless of its location, a lifelong anticoagulation due to abnormal flow conditions is required and the anticoagulation has to be done with VKAs (44). In general, the target INR has to be adapted to the anatomic position of the valve with regularly higher INR levels of the mechanical valve in the atrioventricular position than for the semilunar valves (21,45,46). In addition, there is a difference regarding the recommendation of adding Aspirin between the guidelines. In case of a mechanical heart valve the American guidelines (46), in contrast to the European guidelines (45), recommend adding Aspirin. Thus, despite the long durability of mechanical heart valves these are prone to cardiovascular events and need lifelong anticoagulation with VKA.
In contrast to mechanical heart valves, bioprosthetic heart valves do not require lifelong anticoagulation (45,46). However, there is often the need for repeat replacement of the bioprosthetic valve due to degeneration and thus an additional surgical procedure. Recent results could show that NOACs could as well be safely used in patients with bioprosthetic heart valves and ACHD (22) at least in the short term follow up.
Pregnancy
NOACs have a contraindication during pregnancy and are not allowed to be used (36,47,48). Patients with mechanical heart valves have to receive anticoagulation treatment throughout the pregnancy.
Mechanical heart valves require anticoagulation and thus pose a risk for the mother as well as the fetus. For the mother the most dangerous situation is thromboembolism and/or thrombotic complications of the mechanical valve resulting in stroke needing emergency cardiac surgery or lysis therapy (47,48). The risk is especially increased in patients with older types of heart valves and in particular if there is more than one mechanical heart valve. Complications are elevated when the function of the mechanical valve is impaired before pregnancy and if heparin is used for anticoagulation during the entire pregnancy. From the different anticoagulation schemes proposed, warfarin/phenprocoumon is the safest option for the mother. Many centers try to avoid VKAs the first 6–12 weeks of pregnancy during organogenesis of the fetus. However, VKA associated embryopathy appears to be dose dependent. Below a dose of <5 mg for Warfarin and <3 mg for phenprocoumon, the risk for embryopathies or fetal complications seems to be low (49,50). During the first trimenon low-molecular weight heparin with strict anti-Xa monitoring (target level: 0.8–1.2 U/mL) is commonly recommended. Switch to intravenous heparin is recommended with a mechanical heart valve one week before delivery in hospital setting. In ACHD with essential anticoagulation elective Caesarean section is recommended in order to avoid complications (47,48). The indications and recommended anticoagulation for patients with prosthetic heart valves and in pregnant patients are shown in Table 2.
Table 2
| Underlying disease in ACHD patients | Arrhythmia | Anticoagulation | Target INR |
|---|---|---|---|
| Prosthetic heart valves | |||
| ACHD patient and mechanical heart valve | No influence on anticoagulation scheme | VKA (+ aspirin in American guidelines) | Usually 2.0–3.0 for semilunar valves and 2.5–3.5 for atrioventricular valves |
| ACHD patient and bioprosthetic heart valve | Arrhythmia influences anticoagulation scheme | VKA or NOAC in case of AF/AFl/IART | 2.0–3.0 |
| Otherwise | |||
| VKA or NOAC or only Aspirin for 3 months | |||
| Pregnancy | |||
| ACHD patient and mechanical heart valve | No influence on anticoagulation scheme | VKA (+ Aspirin in American guidelines). For 1 Trimenon LMWH with Anti Xa 0.8–1.2; for 2 and 3 Trimenon VKA (CAVE: VKA below 5 mg per day), switch to heparin 1 week before delivery | Usually 2.0–3.0 for semilunar valves and 2.5–3.5 for atrioventricular valves |
| ACHD patient and bioprosthetic heart valve | Arrhythmia influences anticoagulation scheme | VKA in case of AF/AFl/IART (NOAC is contraindicated). For 1 Trimenon LMWH with Anti Xa 0.8–1.2; for 2 and 3 Trimenon VKA (CAVE: VKA below 5 mg per day), switch to heparin 1 week before delivery | 2.0–3.0 |
| ACHD patient with pregnancy and arrhythmia | Arrhythmia influences anticoagulation scheme | VKA in case of AF/AFl/IART (NOAC is contraindicated). For 1 Trimenon LMWH with Anti Xa 0.8–1.2; for 2 and 3 Trimenon VKA (CAVE: VKA below 5 mg per day), switch to heparin 1 week before delivery | 2.0–3.0 |
ACHD, adults with congenital heart disease; VKA, vitamin K antagonists; NOAC, non-vitamin K oral anticoagulants; AF, atrial fibrillation; AFl, atrial flutter; IART, intra-atrial reentrant tachycardia.
Future implications and limitations of the review
The shown evidence is most commonly based on the respective and actual treatment guidelines, however, currently most of the evidence presented is not from controlled randomized studies but based on larger registries and observational studies. Thus the quality of data is still one of the major limitations in adult patients with congenital heart disease. Planning of randomized trials in a multicenter approach is an essential step to improve quality of care for ACHD patients.
Summary
Anticoagulation in ACHD is a serious issue with increasing importance as the number and complexity of ACHD patients is increasing and many will develop arrhythmias requiring anticoagulation treatment. Traditionally anticoagulation relied on VKA as no alternative was available. Even today prospective randomized controlled trials on the use of NOACs remain to be done in ACHD. Recently, however, international study collaborations even suggested the safety of the NOACs in patients with ACHD in the short term. Considering these observational, non-controlled results, NOACs might be considered in selected indications for ACHD patients. While NOACs might be an option for selected patients, treatment decisions have to be based on the individual case taking into account specific ACHD factors as well the individual risk of stroke and bleeding in particular in cyanotic patients and in those with single ventricles in Fontan circulation. There is certainly a scope for further data and specifically prospective research in this growing, vulnerable patient population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yskert von Kodolitsch, Harald Kaemmerer, Koichiro Niwa) for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part IV” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-631
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-631). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part IV” was commissioned by the editorial office without any funding or sponsorship. SB reports grants and personal fees from Abbott Diagnostics, grants and personal fees from Bayer, grants from SIEMENS, grants from Singulex, grants and personal fees from Thermo Fisher, personal fees from Abott, personal fees from Astra Zeneca, personal fees from AMGEN, personal fees from Medtronic, personal fees from Pfizer, personal fees from Roche, personal fees from Novartis, personal fees from Siemens Diagnostics, outside the submitted work. PK reports non-financial support from European Union, non-financial support from British Heart Foundation, non-financial support from Leducq Foundation, non-financial support from Medical Research Council (UK), non-financial support from German Centre for Cardiovascular Research, outside the submitted work; In addition, Dr. Kirchhof has a patent WO 2015140571 issued, and a patent WO 2016012783 issued and research support for basic, translational, and clinical research projects from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the last three years. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khairy P, Van Hare GF, Balaji S, et al. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm 2014;11:e102-65. [Crossref] [PubMed]
- Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. [Crossref] [PubMed]
- Raissadati A, Nieminen H, Jokinen E, et al. Progress in late results among pediatric cardiac surgery patients: a population-based 6-decade study with 98% follow-up. Circulation 2015;131:347-53; discussion 353. [Crossref] [PubMed]
- Bédard E, Shore DF, Gatzoulis MA. Adult congenital heart disease: a 2008 overview. Br Med Bull 2008;85:151-80. [Crossref] [PubMed]
- Marelli AJ, Ionescu-Ittu R, Mackie AS, et al. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014;130:749-56. [Crossref] [PubMed]
- Marelli AJ, Mackie AS, Ionescu-Ittu R, et al. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 2007;115:163-72. [Crossref] [PubMed]
- Giang KW, Mandalenakis Z, Dellborg M, et al. Long-term risk of hemorrhagic stroke in young patients with congenital heart disease. Stroke 2018;49:1155-62. [Crossref] [PubMed]
- Mandalenakis Z, Rosengren A, Lappas G, et al. Ischemic stroke in children and young adults with congenital heart disease. J Am Heart Assoc 2016;5:e003071. [Crossref] [PubMed]
- Kaemmerer H, Bauer U, Pensl U, et al. Management of emergencies in adults with congenital cardiac disease. Am J Cardiol 2008;101:521-5. [Crossref] [PubMed]
- Kaemmerer H, Fratz S, Bauer U, et al. Emergency hospital admissions and three-year survival of adults with and without cardiovascular surgery for congenital cardiac disease. J Thorac Cardiovasc Surg 2003;126:1048-52. [Crossref] [PubMed]
- Lanz J, Brophy JM, Therrien J, et al. Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation 2015;132:2385-94. [Crossref] [PubMed]
- Hoffmann A, Chockalingam P, Balint OH, et al. Cerebrovascular accidents in adult patients with congenital heart disease. Heart 2010;96:1223-6. [Crossref] [PubMed]
- Budts W, Roos-Hesselink J, Radle-Hurst T, et al. Treatment of heart failure in adult congenital heart disease: a position paper of the Working Group of Grown-Up Congenital Heart Disease and the Heart Failure Association of the European Society of Cardiology. Eur Heart J 2016;37:1419-27. [Crossref] [PubMed]
- Hernández-Madrid A, Paul T, Abrams D, et al. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace 2018;20:1719-53. [Crossref] [PubMed]
- Egbe AC, Connolly HM, McLeod CJ, et al. Thrombotic and Embolic Complications Associated With Atrial Arrhythmia After Fontan Operation: Role of Prophylactic Therapy. J Am Coll Cardiol 2016;68:1312-9. [Crossref] [PubMed]
- Georgekutty J, Kazerouninia A, Wang Y, et al. Novel oral anticoagulant use in adult Fontan patients: A single center experience. Congenit Heart Dis 2018;13:541-7. [Crossref] [PubMed]
- Hijazi Z, Lindback J, Alexander JH, et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J 2016;37:1582-90. [Crossref] [PubMed]
- Oldgren J, Hijazi Z, Lindback J, et al. Performance and Validation of a Novel Biomarker-Based Stroke Risk Score for Atrial Fibrillation. Circulation 2016;134:1697-707. [Crossref] [PubMed]
- Karsenty C, Zhao A, Marijon E, et al. Risk of thromboembolic complications in adult congenital heart disease: A literature review. Arch Cardiovasc Dis 2018;111:613-20. [Crossref] [PubMed]
- Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72. [Crossref] [PubMed]
- Jensen AS, Idorn L, Norager B, et al. Anticoagulation in adults with congenital heart disease: The who, the when and the how? Heart 2015;101:424-9. [Crossref] [PubMed]
- Yang H, Bouma BJ, Dimopoulos K, et al. Non-vitamin K antagonist oral anticoagulants (NOACs) for thromboembolic prevention, are they safe in congenital heart disease? Results of a worldwide study. Int J Cardiol 2020;299:123-30. [Crossref] [PubMed]
- Pujol C, Mussigmann M, Schiele S, et al. Direct oral anticoagulants in adults with congenital heart disease - a single centre study. Int J Cardiol 2020;300:127-31. [Crossref] [PubMed]
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-962. [Crossref] [PubMed]
- Hernandez GA, Lemor A, Clark D, et al. Heart transplantation and in-hospital outcomes in adult congenital heart disease patients with Fontan: A decade nationwide analysis from 2004 to 2014. J Card Surg 2020;35:603-8. [Crossref] [PubMed]
- Khairy P, Aboulhosn J, Broberg CS, et al. Thromboprophylaxis for atrial arrhythmias in congenital heart disease: A multicenter study. Int J Cardiol 2016;223:729-35. [Crossref] [PubMed]
- Dellborg M, Mandalenakis Z. NOACs in adult congenital heart disease - Still limited experience. Int J Cardiol 2020;300:143-4. [Crossref] [PubMed]
- Payne RM, Burns KM, Glatz AC, et al. A multi-national trial of a direct oral anticoagulant in children with cardiac disease: Design and rationale of the Safety of ApiXaban On Pediatric Heart disease On the preventioN of Embolism (SAXOPHONE) study. Am Heart J 2019;217:52-63. [Crossref] [PubMed]
- Mongeon FP, Macle L, Beauchesne LM, et al. Non-Vitamin K Antagonist Oral Anticoagulants in Adult Congenital Heart Disease. Can J Cardiol 2019;35:1686-97. [Crossref] [PubMed]
- Ebrahim MA, Escudero CA, Kantoch MJ, et al. Insights on Atrial Fibrillation in Congenital Heart Disease. Can J Cardiol 2018;34:1531-3. [Crossref] [PubMed]
- Arslani K, Notz L, Zurek M, et al. Anticoagulation practices in adults with congenital heart disease and atrial arrhythmias in Switzerland. Congenit Heart Dis 2018;13:678-84. [Crossref] [PubMed]
- Yang H, Bouma BJ, Mulder BJM, et al. Is Initiating NOACs for Atrial Arrhythmias Safe in Adults with Congenital Heart Disease? Cardiovasc Drugs Ther 2017;31:413-7. [Crossref] [PubMed]
- Wan D, Tsui C, Kiess M, et al. Anticoagulation for thromboembolic risk reduction in adults with congenital heart disease. Can J Cardiol 2017;33:1597-603. [Crossref] [PubMed]
- Faircloth JM, Palumbo JS, Veldtman GR. Overcoming the challenges of anticoagulation in adults with congenital heart disease. Heart 2015;101:418-20. [Crossref] [PubMed]
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955-62. [Crossref] [PubMed]
- Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330-93. [Crossref] [PubMed]
- Giglia TM, Massicotte MP, Tweddell JS, et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease: a scientific statement from the American Heart Association. Circulation 2013;128:2622-703. [Crossref] [PubMed]
- Webb G, Mulder BJ, Aboulhosn J, et al. The care of adults with congenital heart disease across the globe: Current assessment and future perspective: A position statement from the International Society for Adult Congenital Heart Disease (ISACHD). Int J Cardiol 2015;195:326-33. [Crossref] [PubMed]
- Firdouse M, Agarwal A, Chan AK, et al. Thrombosis and thromboembolic complications in fontan patients: a literature review. Clin Appl Thromb Hemost 2014;20:484-92. [Crossref] [PubMed]
- Pinto C, Samuel BP, Ratnasamy C, et al. Thrombosis in Fontan patient on apixaban. Int J Cardiol 2015;182:66-7. [Crossref] [PubMed]
- Cromme-Dijkhuis AH, Henkens CM, Bijleveld CM, et al. Coagulation factor abnormalities as possible thrombotic risk factors after Fontan operations. Lancet 1990;336:1087-90. [Crossref] [PubMed]
- Manlhiot C, Brandao LR, Kwok J, et al. Thrombotic complications and thromboprophylaxis across all three stages of single ventricle heart palliation. J Pediatr 2012;161:513-519.e3. [Crossref] [PubMed]
- Waldmann V, Laredo M, Abadir S, et al. Atrial fibrillation in adults with congenital heart disease. Int J Cardiol 2019;287:148-54. [Crossref] [PubMed]
- Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013;369:1206-14. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521-643. [Crossref] [PubMed]
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165-241. [Crossref] [PubMed]
- Canobbio MM, Warnes CA, Aboulhosn J, et al. Management of Pregnancy in Patients With Complex Congenital Heart Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2017;135:e50-e87. [Crossref] [PubMed]
- D'Souza R, Ostro J, Shah PS, Silversides CK, et al. Anticoagulation for pregnant women with mechanical heart valves: a systematic review and meta-analysis. Eur Heart J 2017;38:1509-16. [Crossref] [PubMed]
- Hassouna A, Allam H. Limited dose warfarin throughout pregnancy in patients with mechanical heart valve prosthesis: a meta-analysis. Interact Cardiovasc Thorac Surg 2014;18:797-806. [Crossref] [PubMed]


