Impact of statins and beta-blocker therapy on mortality after coronary artery bypass graft surgery
Introduction
Coronary artery disease (CAD) is a marker of significant atherosclerotic disease and establishes a population at increased risk for recurrent ischemic events including stroke and myocardial infarction (MI) (1,2). Coronary artery bypass graft surgery (CABG) is an effective treatment strategy for severe CAD but its long-term effectiveness is limited by the progression of native and bypass conduit atherosclerosis (3). Ten years after CABG, only 60% of vein grafts are patent and patent grafts show moderate atherosclerotic change (4). Thus, treatment of the underlying atherosclerotic risk factors with attention to blood pressure control, glycemic control and lipid lowering becomes an essential long-term strategy. Statins are an essential component of such an approach as they exert lipid lower actions and reduce low density lipoproteins (LDL), retard the progression of atherosclerosis and improve survival by reducing the risk of vascular death, non-fatal MI and stroke (5). Statins also exert non-lipid-related actions including improvement in endothelial function, nitrous oxide (NO) levels, antioxidant activity and reduce neointimal formation and smooth muscle proliferation (6).
The current national secondary prevention guidelines currently recommend treatment to achieve LDL levels <70 mg/dL for patients with documented atherosclerotic vascular disease, including patients after CABG (7). Despite, these established treatment standards, statin therapy is underutilized among patients undergoing CABG. Even in the context of the SYNTAX trial (synergy between percutaneous coronary intervention with taxus and cardiac surgery) only 74% of patients received statins after CABG (8).
We conducted a retrospective cohort study of patients after first-time isolated CABG and assessed the impact of a discharge regimen including beta-blockers and statin therapy and their relationship to long-term all cause mortality and major adverse cardiovascular events (MACE).
Patients and methods
Patients
We identified patients age >18 years, undergoing first time isolated CABG from 1993 to 2005 with complete clinical, laboratory data and follow-up data. We excluded patients (<18 years and those undergoing repeat open heart surgeries). Patients were identified using the Cardiovascular Information Registry (CVIR). This registry contains detailed demographic, clinical, pathologic, operative, and outcome variables on all patients undergoing cardiac surgery at Cleveland Clinic, abstracted from clinical records concurrent with patient care. We collected follow-up information at 30, 60, 90 days and yearly follow-up. The registry is approved for use in research by the institutional review broad.
Definitions
Stroke was defined as an acute loss of focal neurological function lasting >72 h due to ischemia. MI was defined as requiring (ischemic symptoms, EKG changes and or ST-T changes or the development of q-waves, or the elevation of cardiac biomarker 10× the upper limit of normal).
Endpoints
Individual primary endpoints include all cause mortality, stroke and MI. Secondary endpoints included MACE (defined as the combined endpoint of death, stroke and MI).
Data analysis
Demographic data were compared between groups using the χ2 test for categoric variables, and continuous variables were compared using the t-test. Multiple logistic regression analyses for predictive factors affecting mortality were carried out in four steps. In the first step, a likelihood ratio backward stepwise approach was used to test the demographic variables. Statistically significant variables (P<0.05) were kept in the model for the second step. In this second step, a similar approach was used to analyze medical history variables [diabetes mellitus, complicated diabetes mellitus, peripheral vascular disease, family history of CAD, carotid disease, all-arterial graft, type of grafts (vein, radial, mammary), number of grafts, chronic obstructive pulmonary disease, previous pulmonary emboli, preoperative ventricular or atrial tachycardia]. Statistically significant variables were kept in the model for the third step in which preoperative data (angina, recent myocardial infarct, recent stroke, heart failure) were tested in a similar approach. Finally, post-operative drug treatments, including two-way interactions between the four types of medications (beta-blockers, ace-inhibitors, statins and antiplatelets agents), were tested in a similar approach conditional to the presence of variables kept in the model from the previous steps.
Results
Patient cohort
We identified 5,205 patients and collected complete information on 3,637 patients with complete follow-up data who underwent single isolated CABG between January 1993 and December 2005. The mean age was 64.5±9.7 years and over 70% were male. And 50% of the patients discharged after CABG were on statin therapy but we had access to 768 patients who were prescribed statins. The median follow-up for the entire cohort was 11 years. Overall, 119 patients had a stroke, 75 patients had a MI and 629 patients died after CABG.
Baseline characteristics
There were no differences in the baseline characteristics with regard to demographics or co-morbidities between those who did (n=768) and did not (n=2,869) take statins at the time of discharge (Table 1). More patients who were on statins were also on ace-inhibitors, beta-blockers and antiplatelet therapy. There was no significant difference in the total cholesterol between the two groups. There was a significant difference in the LDL-C concentration between the two groups [134±41.9 mg/dL (no statin) vs. 126±44.8 mg/dL (with statin), P=0.001]. There were a significantly larger proportion of patients who had a lower LDL-C who were on statin therapy for each LDL strata.
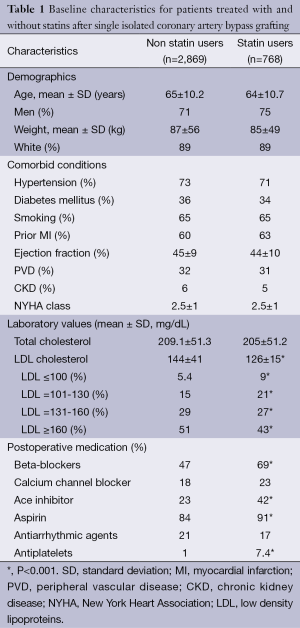
Full table
Impact of statin use at discharge on primary and secondary endpoints
In the multivariate Cox regression analysis is shown in Table 2 after adjustment for sex, weight, age, ejection fraction atrial fibrillation, hypertension, smoking, peripheral vascular disease and medications received during follow-up (including antiplatelets, angiotensin converting enzyme inhibitors, beta-blockers and statins). The hazard ratios (HR) for the individual endpoints of death, MI or stroke at 1 year were 0.424 (95% CI: 0.26-0.68, P<0.001), 1.8 (95% CI: 0.5-6.0, P=0.322) and 1.04 (95% CI: 0.34-3.17, P=0.937) respectively. The overall HR for death, MI and stroke were 0.69 (95% CI: 0.34-3.176, P=0.001), 1.2 (95% CI: 0.7-2.0, P=0.49) and 0.87 (95% CI: 0.54-1.4, P=0.30) respectively. We note that the predictors of overall death include no therapy with statin therapy, age (HR 1.1, 95% CI: 1.04-1.078, P<0.001), and history of diabetes mellitus, prior MI, carotid disease and renal dysfunction. Overall death was also significantly reduced in patient’s who were concurrently prescribed beta blockers inhibitors (HR 0.79, 95% CI: 0.6-0.9, P=0.007), aspirin (HR 0.43, 95% CI: 0.35-0.52, P<0.001) and calcium channel blockers (HR 0.79, 95% CI: 0.63-0.98, P=0.034).
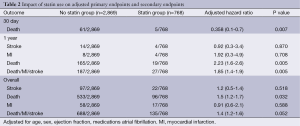
Full table
Impact of statin use on MACE
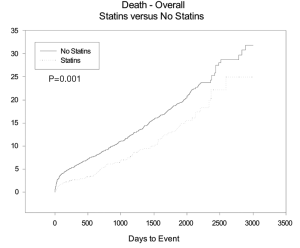
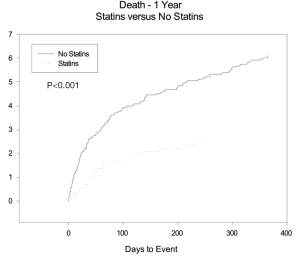
The HR for MACE overall and at 1 year were 0.76 (95% CI: 0.63-0.92, P=0.005) and 0.5 (95% CI: 0.35-0.8, P=0.003). MACE was also significantly reduced in patient’s who were discharged on aspirin and beta blockers. The estimated 11-year Kaplan Meier curves for curves for mortality between the two groups starts to diverge immediately post discharge after single isolated CABG and continue to diverge through out the follow-up period see Figure 1.
LDL-C cholesterol level and primary and secondary endpoints
Patients with an LDL-C level ≤100 mg/dL in comparison to LDL-C ≥130 mg/dL and LDL >160 mg/dL were noted to have a significantly lower incidence of death (HR 0.60, 95% CI: 0.39-0.9, P=0.027), MI (HR 0.26, 95% CI: 0.087-0.78, P=0.008) and the composite endpoint of death, MI and stroke (HR 0.65, 95% CI: 0.45-0.92, P=0.014). There were no significant differences in the incidence of stroke based on the LDL-C strata as shown in Table 3.
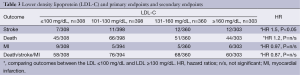
Full table
Impact of statin therapy on mortality with respect to ejection fraction
The overall (5.8%) and 1 year mortality (20.1%) was significantly higher in patients with post-operative left ventricular dysfunction who were not on statins (P<0.001) as shown in Table 4. In the patients who were prescribed statins, there was a significantly higher incidence of death at 1 year (3.2% vs. 2.1%, P=0.026) in patients with post-operative left ventricular dysfunction. However, there was no statistically significant difference in overall mortality in patients treated with statins with (12.8%) or without left ventricular dysfunction (9.2%), P=0.35.
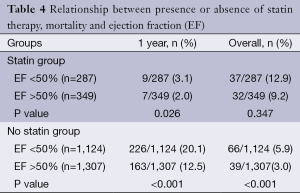
Full table
Impact of medications on mortality
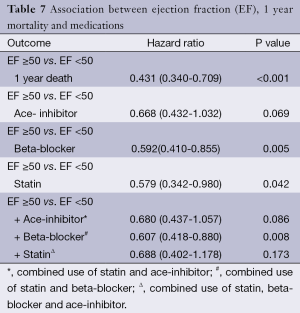
The use of statins and beta-blockers were associated with a significantly lower incidence of overall death (HR 0.69, 95% CI: 0.559-0.867, P=0.001; HR 0.73, 95% CI: 0.62-0.85, P<0.001 respectively) as shown in Table 5. There was a significantly lower incidence of one year mortality with the use of statins (HR 0.42, 95% CI: 0.26-0.68, P<0.001), beta-blockers (HR 0.438, 95% CI: 0.467-0.960, P<0.001) and ace-inhibitors (HR 0.67, 95% CI: 0.47-0.69, P=0.029) (Figure 2). There was a significant reduction in overall death and 1 year death in the subgroups of patients with and without statins with normal or low ejection fractions as shown in Tables 6 and 7. The impact of statins and beta-blockers on mortality was additive at 1 year (P<0.001) and at 11 years (P=0.002) as shown in Table 8.
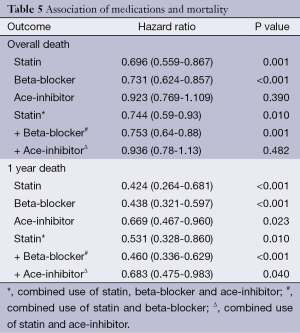
Full table
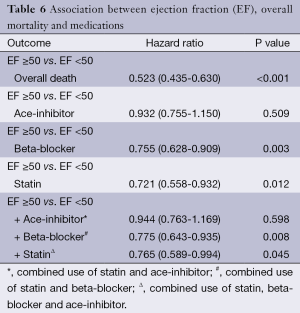
Full table
Full table

Full table
Discussion
In this study, we show that a discharge regimen, which adheres to the current standards for secondary prevention including antiplatelet agents, statins drugs are associated with a reduction in mortality, MI rates and major adverse cardiac events. The following observations can be made with regard to the contributions of individual drugs.
A post-operative discharge regimen including statins was associated with a significant reduction in mortality by 57% and 31% mortality at 1 year and 11 years respectively. This benefit was independent of other predictors of mortality including age, concomitant baseline differences in prescribed medications and baseline character tics of the patients. The unadjusted rates for death, stroke, MI were still significantly lower despite the difference in baseline characteristics suggesting patients on statin therapy were an overall sicker group (with significantly more diabetes mellitus and peripheral vascular disease). Patients on statin medication also had significantly lower LDL-C between the two groups (P=0.001) and there were a significantly larger proportion of patients who had a lower LDL-C who were on statin therapy for each LDL strata. Serum LDL-C levels ≤100 mg/dL in comparison to LDL-C ≥130 mg/dL was associated with a significantly lower incidence of overall death (HR 0.60, 95% CI: 0.39-0.9, P=0.027), MI (HR 0.26, 95% CI: 0.087-0.78, P=0.008) and the MACE (HR 0.65, 95% CI: 0.45-0.92, P=0.014). A post-discharge regimen of statins after CABG reduces mortality though its effects on lowering of serum LDL-C levels.
These data are consistent with the randomized trials, which have evaluated the role of cholesterol reduction following CABG. The Cholesterol-Lowering Atherosclerosis Study (CLAS) trial, LOpid and Coronary Atherosclerosis (LOCAT) and post CABG trial have shown an association between lowering serum LDL-C and retarding the progression of native atherosclerosis (LOCAT), reduction in vein graft occlusion (CLAS, LOCAT) and reduction in the quadruple ischemic and revascularization endpoints (LOCAT, Post CABG trial) (9-14). There are several limitations to these studies including delayed initiation of lipid lowering therapy (1-11 years after CABG), statin therapy was used on only one trial (post-CABG trial), patient population consisted of a largely young population without significant co-morbidities, short duration of follow-up (2 years in CLAS to 7.5 years in the post CABG trial) and use of quadruple endpoints in all three studies with no trial demonstrating a reduction in mortality associated with statin use.
More contemporary data has become available from post hoc subgroup analysis of contemporary randomized controlled trials. The Cholesterol and Recurrent Events (CARE) trial subgroup of patients who had undergone CABG in the last 3 months, pravastatin reduced the all cause mortality by 4.4% and fatal MI was reduced by 3.2% (15). Similarly, the sub group analysis of the Treating to New Targets (TNT) trial involving 4,654 patients with previous CABG, the primary outcome (defined as fatal or nonfatal MI, stroke or resuscitated cardiac arrest) in 9.7% (atorvastatin 80 mg) vs. 15% (atorvastatin 10 mg) (HR 0.73, 95% CI: 0.62-0.87, P=0.0004) or repeat revascularization was needed and 11% (atorvastatin 80 mg) vs. 16% (atorvastatin 10 mg) (HR 0.70, 95% CI: 0.60-0.82, P<0.0001) (16). We similarly show a significant reduction in overall death and a non-significant trend in reduction MACE (P=0.052).
Some observational studies also confirmed a reduction is quadruple composite of ischemia and revascularization and an independent association between early statin use within a month of CABG discharge and a reduction in the risk of all cause mortality (adjusted HR 0.82, 95% CI: 0.72-0.9, P=0.004) (17). We are able to support such findings and show a mortality difference in the statin group vs. no statin group (HR 0.358, 95% CI: 0.122-0.758, P=0.007) at 30 days and at the end of follow-up (11 years).
Our data suggests a discharge regimen of statins early post CABG and supports the current existing American Heart Association/American College of Cardiology (AHA/ACC) secondary prevention guidelines. This is particularly important as only 7% of patients with atherosclerosis are able to achieve LDL levels less than 100 mg/dL with diet and exercise regimens, in the absence of severe contraindications, essentially all CABG patients are candidates for long-term postoperative statin therapy (8). Nevertheless, statin use underutilized in the post CABG population. Even in the context of randomized controls studies, only 74% of patients in the recent Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) trial received statins after CABG (18). The most recent AHA/ACC guideline update for CABG states that all patients undergoing CABG should receive statin therapy unless otherwise contraindicated (class I, level A) (7). No mention is made in this or any guideline or statement regarding the optimal dose of and timing of statin administration in the postoperative period. However, withholding statins until after surgical discharge may adversely affect compliance. Several studies have reported the highest adherence rates to therapy and the greatest likelihood of achieving target LDL levels are if statins were initiated before hospital discharge following a cardiovascular event (19).
Additional findings of the present study included the association between post-operative statins and beta-blockers acting in a synergistic and additive manner in reducing overall and 1 year mortality as shown in Table 8. This association remains true in patients irrespective of post-CABG left ventricular dysfunction as shown in Tables 6 and 7. We also note that while the presence of left ventricular dysfunction very strong predictor of overall and 1-year mortality in those not prescribed statin medications. The presence of left ventricle dysfunction is no longer significantly associated with overall mortality in patients treated with statins.
Although the AHA/ACC guidelines recommend long-term aspirin and statin therapy in all patients after CABG, their comments on beta-blockers use are limited to encouraging their use in the immediate preoperative period to reduce the risk of atrial fibrillation (7). These recommendation are based on a the only randomized controlled trial to evaluate the long-term use of beta-blocker therapy after CABG, the metoprolol after coronary bypass (MACB) study demonstrated that 100 mg of metoprolol twice per day for 2 years after surgery did not reduce the incidence of repeat revascularization, unstable angina, non-fatal MI, or death compared with placebo (20). Likewise, a secondary analysis of data from the Project of ex-vivo Vein Graft Engineering via Transfection IV (PRE-VENT IV) trial failed to demonstrate any significant association between beta-blockers use at the time of discharge and the rate of death or MI in the 2 years after CABG (21). However, these negative results were based on small numbers (81 cardiac events in the MACB trial and 147 deaths or MI in PREVENT IV).
There is a plethora of evidence to suggest that beta-blockers improve mortality after myocardial infraction (60% of our patients). A meta-analysis of 54,234 patients entered into 82 randomized trials found that short-term beta blockade immediately after an acute MI was unlikely to be of major benefit unless treatment was discontinued long-term (22). A more contemporary evaluation of the potential benefit from long-term beta-blocker use was made in a 2012 observational study of over 14,000 patients with MI enrolled in the international REACH registry. Patients were enrolled in 2003 and 2004 and followed prospectively for up to 4 years. The primary outcome was a composite of cardiovascular death, nonfatal MI, or nonfatal stroke. After a median follow-up of 44 months, there was no significant difference in the primary outcome (16.9% vs. 18.6%, respectively; HR 0.90, 95% CI: 0.79-1.03). Little difference was seen in the event rates in the beta blocker and no beta-blocker groups after 2 years (23). Our data adds to the current body of evidence that secondary prevention initiatives should be promoted and adherence emphasized after CABG as they reduce overall and 1 year mortality. We also have shown for the first time a synergistic and additive effect of statins and beta-blockers on mortality.
Our study has several limitations. Firstly, our study is observational in nature and despite adjustment for confounders in the multivariate analysis we can only investigate associations and cannot infer causality. Secondly, we only have information on medication that was prescribed at the time of discharge and cannot verify continued use.
In conclusion, a post-discharge regimen of statins independently reduces overall and 1 year mortality. These results confirm those of earlier studies within a contemporary surgical population and support the current clinical guidelines. Clearly, more research is needed to increase statin use among CABG patients, including quality improvement initiatives focused on the prescription habits of cardiac surgeons and cardiologists. Several studies have explored approaches to solving these treatment gaps in cardiovascular prevention. The evaluation and implantation of such quality improvement initiative post CABG will undoubtedly increase statin prescription rates in these patients who, despite surgical revascularization, remain at risk for future cardiovascular events.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78. [PubMed]
- Rossouw JE. Lipid-lowering interventions in angiographic trials. Am J Cardiol 1995;76:86C-92C. [PubMed]
- Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994;344:563-70. [PubMed]
- Domanski MJ, Borkowf CB, Campeau L, et al. Prognostic factors for atherosclerosis progression in saphenous vein grafts: the postcoronary artery bypass graft (Post-CABG) trial. Post-CABG Trial Investigators. J Am Coll Cardiol 2000;36:1877-83. [PubMed]
- Armitage J. The safety of statins in clinical practice. Lancet 2007;370:1781-90. [PubMed]
- Calabrò P, Yeh ET. The pleiotropic effects of statins. Curr Opin Cardiol 2005;20:541-6. [PubMed]
- Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation 2004;110:e340-437. [PubMed]
- Goldsmith I, Lip GY, Emsden K, et al. Hyperlipidaemia management after primary coronary artery bypass surgery: a survey of patients and general practitioners. J Cardiovasc Risk 1999;6:263-7. [PubMed]
- Lazar HL. Role of statin therapy in the coronary bypass patient. Ann Thorac Surg 2004;78:730-40. [PubMed]
- Campeau L. Failure of saphenous vein coronary artery bypass grafts and its potential prevention. Curr Opin Cardiol 1987;2:990-5.
- Blankenhorn DH, Nessim SA, Johnson RL, et al. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA 1987;257:3233-40. [PubMed]
- Cashin-Hemphill L, Mack WJ, Pogoda JM, et al. Beneficial effects of colestipol-niacin on coronary atherosclerosis. A 4-year follow-up. JAMA 1990;264:3013-7. [PubMed]
- Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med 1997;336:153-62. [PubMed]
- Frick MH, Syvänne M, Nieminen MS, et al. Prevention of the angiographic progression of coronary and vein-graft atherosclerosis by gemfibrozil after coronary bypass surgery in men with low levels of HDL cholesterol. Lopid Coronary Angiography Trial (LOCAT) Study Group. Circulation 1997;96:2137-43. [PubMed]
- Flaker GC, Warnica JW, Sacks FM, et al. Pravastatin prevents clinical events in revascularized patients with average cholesterol concentrations. Cholesterol and Recurrent Events CARE Investigators. J Am Coll Cardiol 1999;34:106-12. [PubMed]
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425-35. [PubMed]
- Kulik A, Brookhart MA, Levin R, et al. Impact of statin use on outcomes after coronary artery bypass graft surgery. Circulation 2008;118:1785-92. [PubMed]
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72. [PubMed]
- Williams JB, Delong ER, Peterson ED, et al. Secondary prevention after coronary artery bypass graft surgery: findings of a national randomized controlled trial and sustained society-led incorporation into practice. Circulation 2011;123:39-45. [PubMed]
- Sjöland H, Caidahl K, Lurje L, et al. Metoprolol treatment for two years after coronary bypass grafting: effects on exercise capacity and signs of myocardial ischaemia. Br Heart J 1995;74:235-41. [PubMed]
- Mehta RH, Ferguson TB, Lopes RD, et al. Saphenous vein grafts with multiple versus single distal targets in patients undergoing coronary artery bypass surgery: one-year graft failure and five-year outcomes from the Project of Ex-Vivo Vein Graft Engineering via Transfection (PREVENT) IV trial. Circulation 2011;124:280-8. [PubMed]
- Freemantle N, Cleland J, Young P, et al. beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999;318:1730-7. [PubMed]
- Bangalore S, Steg G, Deedwania P, et al. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA 2012;308:1340-9. [PubMed]


