
Chest radiography of contemporary trans-catheter cardiovascular devices: a pictorial essay
Introduction
There is a plethora of newer transcatheter intra-cardiac and vascular devices. Chest radiography (CXR) plays an important role in assessment and follow-up of such devices. It is often the initial imaging test ordered after placement, though many devices are incidentally discovered on CXR. This pictorial essay aims to highlight the radiographic appearances of relevant, recent cardiovascular devices that are deployed using a percutaneous, trans-catheter technique. We discuss appropriate indications for usage, complications related to abnormal placement, MR compatibility, and closest mimics of each device.
Classification
Transcatheter cardiovascular devices can be categorized based on functionality:
- Valvular pathologies: transcatheter aortic and pulmonic valve replacement (TAVR, TPVR) and percutaneous management of mitral as well as tricuspid regurgitation (TR) (clip placement);
- Congenital heart disease: atrial and ventricular septal occlusion, patent ductus arteriosus (PDA) closure, and endovascular treatment for coarctation of aorta;
- Heart failure and post myocardial infarction (MI): ventricular partitioning device is highlighted. Transcatheter ventricular assist devices, intra-aortic balloon pumps (IABP) placed via femoral or axillary arteries, and different configurations of extra-corporeal membrane oxygenation cannulae (ECMO) are also shown;
- Monitoring and pacing: leadless cardiac pacemaker, implantable loop recorder, and ambulatory heart monitoring devices are highlighted. An exhaustive list of various routine pacemaker and cardiac defibrillator devices as well as the now common Swan-Ganz catheter are not included in this review, though some newer lead configurations such as Bundle of His leads which are positioned towards the IVS are shown;
- Prevention of thromboembolism in the systemic and pulmonary circulation: Percutaneous left-atrial appendage closure and cava filters.
Discussion
An increasing array of cardiovascular diseases are amenable to trans-catheter management, and newer trans-catheter devices are being constantly introduced or modified. Continuously improving efficacy and safety of these procedures have widened the target population to include not only candidates who are medically unfit for surgery but also selected patients who have no contraindications for surgery. Trans-catheter procedures are less invasive, with many offering comparable efficacy when compared to surgery (1). Some procedures such as trans-catheter valve replacement and closure of septal defects have become commonplace in interventional cardiology, while others such as left ventricular partitioning device placement, are relatively uncommon.
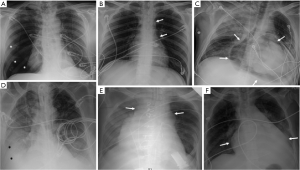
CXR is often the initial imaging screening test to identify and evaluate such devices, and thus plays a crucial role. It can provide easy confirmation of optimal device placement and help in early detection of potentially grave complications related to abnormal placement before these are manifested clinically. Imagers should therefore be familiar with these devices, their proper imaging appearances and potential complications. Malpositioning, migration/embolization and fracture/deformity of cardiovascular devices, as well as post-procedure complications such as pneumothorax (Figure 1A), pneumomediastinum (Figure 1B), pneumopericardium (Figure 1C), and hemorrhage within pleural (Figure 1D), mediastinal (Figure 1E), or pericardial compartments (Figure 1F) resulting in new/enlarging/loculated pleural effusions, cardio-mediastinal widening, and pericardial effusion are radiographically-detectable. There are inherent limitations of the CXR in detecting all potential complications related to such devices. Several device-specific complications such as infection, thromboembolism, cardiac arrhythmias, myocardial or vessel wall trauma, etc., are not detectable by CXR. We emphasize that clinical suspicion of such radiographically-occult complications should prompt further imaging with echocardiography or computed tomography (CT).
Echocardiography and CT are not indicated as routine screening tests for device placement though may serve as important problem solving tools to confirm abnormal placement and further evaluate suspected complications. For example, CXR obtained after a pacemaker implantation can suggests abnormal position of a pacemaker lead outside the cardiac borders. A subsequent echocardiogram can show tamponade from myocardial perforation, while CT is able accurately pin point the abnormal location of the lead, site of tear, etc. A magnetic resonance imaging (MRI) scan may be indicated in patients with indwelling devices for evaluating underlying cardiac or other unrelated diseases. This may prompt a screening CXR to “clear” these patients for MRI, and thus knowledge of MR compatibility of each device is important.
Trans-catheter intra-cardiac and vascular devices have characteristic radiographic appearances. Several of these devices remain connected to long intra-arterial or intravenous catheters, with a portion projecting into the cardiac chambers. Examples include temporary transcatheter ventricular assist device or Impella (tip inside the ventricular cavity), IABP (tip within the proximal descending aorta), ECMO cannulae (tip in the vena-cavae or right atrium for veno-venous, and in a central or peripheral artery and vein in veno-arterial), and Swan-Ganz catheters (tip in a central pulmonary artery- usually the right). Cardiac pacemakers and defibrillators with chest-wall pulse-generator and intra-cardiac leads are fairly common, though a few newer lead positions (Bundle of His) may not be routinely encountered.
Most other relevant transcatheter devices have one of few distinct shapes, namely—stent-like, short clip-like, capsular appearance and clamshell, umbrella or parachute shaped. The smallest of the reviewed devices (CardioMEMS) has a unique figure-of-eight shape.
Some of these devices are seen in characteristic locations such as the SVC area, distal aortic arch and aorto-pulmonary window region, which make them easier to recognize on a single view radiograph (frontal or PA). Other devices which project over specific chambers, septae or valves, can be difficult to accurately localize on a single frontal or PA view without the help of a lateral CXR.
Valvular pathologies
Transcatheter aortic valve devices
Clinical indications
Since 2002, TAVR has been validated as a suitable alternative to surgical aortic valve replacement in selected patients with severe aortic stenosis considered non-operable or intermediate- high risk for surgical repair (2,3).
Device make and radiographic appearance
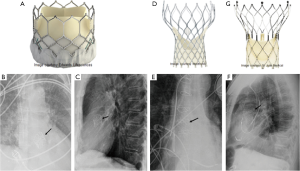
Commercially available TAVR systems include the Sapien 3 (Edwards Lifesciences, Irvine, CA), Sapien XT (Edwards Lifesciences, Irvine, CA), CoreValve (Medtronic Inc., Minneapolis, MN), and Portico (St Jude Medical or Abbott Inc., Chicago, IL) trans-catheter aortic heart valves. The Sapien 3 valve (Figure 2A,B,C) and Sapien XT valve have similar constructions and use bovine pericardial tissue fixed to a cobalt chromium alloy frame and capped with a polyethylene outer skirt. The CoreValve is made from porcine cardiac tissue and self-expanding nitinol frame (Figure 2D,E,F). The Portico valve has bovine leaflets with a porcine sealing cuff and flared self-expanding stent (Figure 2G). On CXRs, each of these trans-catheter valves with their different designs are localized to the aortic valve position (4).
Device-specific complications
Annular rupture (i.e., injury to the aortic root and/or left ventricular outflow tract) is the most severe complication. Stent fracture and device malposition, migration and/or embolization are other potential complications (5,6).
Transcatheter pulmonary valve devices
Clinical Indications
TPVR is used for pulmonic regurgitation or pulmonic stenosis (7,8).
Device make and radiographic appearance
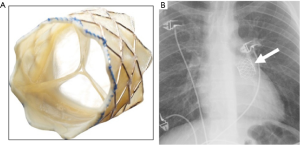
TPVR devices are usually made from bovine or porcine tissue and multiple diameters (sizes) are available. Melody pulmonic valve (Medtronic Inc., Minneapolis, MN) is a commercially available trans-catheter pulmonary valve and consists of a bovine jugular venous conduit that is attached to a balloon-expandable platinum stent (Figure 3A) (9). On CXRs, the Melody valve metallic stent should be projected in the position of the pulmonic valve (Figure 3B).
Device-specific complications
Non visualization of a Melody valve in the expected position may indicate malposition, migration, or embolization. Stent fracture can also be noted at radiography. Additional complications include paravalvular leak, pulmonary artery pseudo-aneurysm and pulmonary hemorrhage (10).
Percutaneous mitral regurgitation therapy (mitral clips)
Clinical indications
Percutaneous mitral valve repair using the MitraClip system (Abbott Structural Heart, Santa Clara, CA) creates a double orifice and has been successfully used in selected patients with functional or degenerative mitral regurgitation (11).
Device make and radiographic appearance
The MitraClip is a polyester-covered cobalt chromium clip. Two adjacent metallic clips in the expected mitral position are seen on radiography (Figure 4A,B,C) (4).
Device-specific complications
Device complications include injury to the mitral valve, partial clip detachment and embolization, device migration (non-mitral position), and mitral stenosis (12,13).
Percutaneous TR therapy (tricuspid clips)
Clinical indications
Symptomatic TR has been treated with off-label use of MitraClip system, though clips specifically designed for the tricuspid valve (TriClip) have recently been FDA approved to treat symptomatic severe TR (Abbott Structural Heart, Santa Clara, CA) (14).
Device make and radiographic appearance
Similar to the MitraClip though in a tricuspid location (midline on frontal CXR, Figure 4D).
Mimic
Per-oral endoscopic myotomy clips used for endoscopic management of achalasia may look similar to MitraClip or Triclip. The myotomy clips project either midline or to the right of midline in keeping with the anatomic location of a dilated thoracic esophagus.
Congenital heart disease
Atrial septal defect (ASD) occlusion device
Clinical indication
These are indicated in ASD with right atrial and right ventricular enlargement, left-to-right shunting, prevention of paradoxical embolisms, and documented platypnea-orthodeoxia syndrome. Only the medium to small secundum ASDs are amenable to percutaneous closure (15,16).
Device make and radiographic appearance
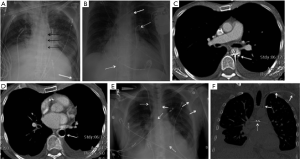
The Amplatzer Septal Occluder (St Jude Medical or Abbott Inc., Chicago, IL) is a catheter-based ASD closure device. It is composed of self-expanding circular double-disk nitinol mesh that snugly occupies the defect with the disc rims closing both sides of the septal wall (Figure 5A) (17). Multiple sizes are available and it may also be used for ventricular septal defect (VSD) closure. On radiographs, the rounded nitinol disks should be projected over the expected site of the interatrial septum or over the interventricular septum in cases of VSD closure (Figure 5B,C,D).
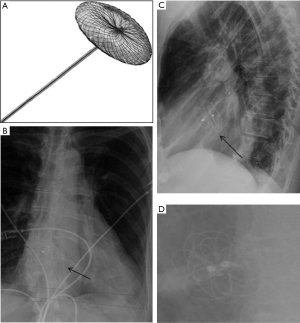
Device-specific complications
Device embolization is suggested if the device is not seen over its expected location either in the interatrial or interventricular septal region. Residual shunts seen on echocardiography and thromboembolic events are potential complications of a failed procedure. Device infections, arrhythmias and cardiac perforations (rare) can also occur.
Patent ductus arteriosus (PDA) closure devices
Clinical indications
Trans-catheter occlusion of PDA may be indicated for non-surgical patients with left ventricular volume overload, patients with pulmonary artery hypertension (PAH) but with pulmonary artery pressure (PAP) <2/3 of systemic pressure or pulmonary vascular resistance (PVR) less than 2/3 of systemic vascular resistance (SVR), and small PDAs with continuous murmur (18).
Device make and radiographic appearance
Multiple devices such as Amplatzer duct occluder II (ADO) devices (St Jude Medical or Abbott Inc., Chicago, IL) (Figure 6), Cera occluder device, and coils have been used. For larger defects other devices include Cardi-O-Fix device, Cocoon device and muscular septal defect occluder. Recently, a newer device (Occlutech occluder, Occlutech Germany) was introduced with designs to overcome some of the limitations of the ADO device. These devices are seen in the aorto-pulmonary window on radiography (19,20).
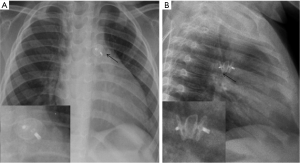
Device-specific complications
Device embolization, infection, narrowing of the aortic isthmus and left pulmonary artery, hemolysis, spontaneous recanalization, and post-procedure left ventricular systolic dysfunction are potential complications (21).
Endovascular treatment for coarctation of aorta
Clinical indication
A primary therapeutic option for many adults and adolescents with coarctation of the aorta, or re-coarctation at the site of prior repair, is intravascular stent therapy (22).
Device make and radiographic appearance
The most often used devices are balloon-mounted stents such as Palmaz (Cordis, Miami Lakes, FL) or Cheatham Platinum (NuMed, Hopkinton, NY) (18,19,23,24). On CXRs, the slightly curved metallic stent is visualized in the expected location of the coarctation, along the distal aortic arch/proximal descending aorta (Figure 7), and rarely characteristic rib notching may be seen (due to chest wall collateral circulation).
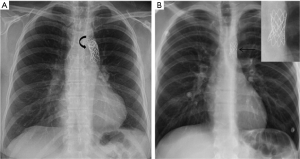
Device-specific complications
Stent fracture is well recognized but usually does not lead to adverse outcome. Recurrent narrowing is rare. Stent migration during implantation is a potential complication, due to stent slippage or balloon rupture. True late migration may be extremely rare, although fragment distal embolization can be seen with circumferential stent fracture (25,26).
Heart failure and post MI
Ventricular Partitioning Device (Cardiokinetix, Menlo Park, CA)
Clinical indication
To preserve normal left ventricular morphology and prevent post injury MI remodeling, reduce left ventricular end diastolic volume, and isolate nonfunctioning from functioning myocardium (27).
Device make and radiographic appearance
The parachute-shaped or umbrella-like device was developed to restore left ventricular function in patients with heart failure and LV apical aneurysm after MI. It is placed percutaneously, and composed of fluoropolymer membrane fitted to an umbrella-shaped nitinol frame. The device “foot” should be seated at the left ventricular apex (Figure 8) on radiographs (28).
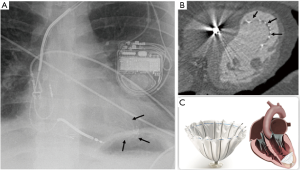
Device specific complications
Device malposition or migration are possible complications. Thromboembolism may contribute to a reported stroke rate of 15.9% at 3 years (29).
Catheter based devices for mechanical circulatory support
The Impella (Abiomed) is a transcatheter ventricular assist device used for short-term mechanical circulatory support in the setting of cardiogenic shock. It comprises of an inflow (impeller) which draws in blood from the ventricle and an outflow into the ascending aorta. It is inserted percutaneously through the femoral (or axillary) artery and advanced retrograde through the aorta with its bent tip snugly inside the left ventricular cavity (Figure 9). A similar device (Impella RP) can be used for right-sided or biventricular failure (4). The Impella device is unsafe for MRI imaging (30).
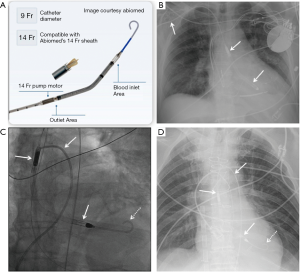
IABP counterpulsation is a form of temporary mechanical hemodynamic support. It comprises of a long catheter inserted percutaneously through the femoral (or axillary) artery and advanced retrograde through the aorta with its tip in the proximal descending thoracic aorta. The distal radiopaque marker should project over the proximal descending aorta (Figure 10) (31). This is unsafe for MR imaging (30).
ECMO is used for cardiorespiratory support and can be of two types: veno-venous (VV) and veno-arterial (VA). VV is primarily used for gas exchange (respiratory support) and VA provides both gas exchange and hemodynamic support. CXR shows wide bore venous catheters in the vena-cavae or right atrium for VV or in a central vein and artery/aorta for VA configurations (Figure 11) (31).
The most serious complications related to these catheter based devices include injury to vessel wall, myocardial wall perforation, thrombus formation and catheter fracture. Disruption of the mitral valve apparatus with resultant severe mitral regurgitation has also been reported with the Impella.
Monitoring and pacing
Leadless cardiac pacemakers
Clinical indications
Variety of cardiac arrhythmias and morphologic abnormalities are considered as indication for cardiac pacing (32). Newly developed leadless pacemakers can be used for patients who meet the criteria for right ventricular pacing. These devices have the advantage of avoiding lead-associated complications such as lead dislodgement or fracture, cardiac perforation, or venous thrombosis and allow for reliable performance and improved safety (33,34).
Device make and radiographic appearance
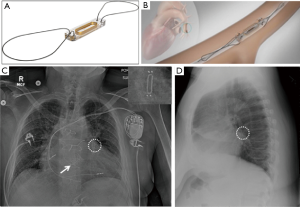
The Nanostim Leadless Pacemaker System (St. Jude Medical or Abbott Inc., Chicago, IL) and the Micra Transcatheter Pacing System (Medtronic Inc., Minneapolis, MN) are the current leadless pacemakers. Both are delivered percutaneously to the right ventricle and are transfixed to the myocardium at the apex.
They have screw-in fixation electrode, or multiple tiny nitinol tines that hold the devices in place. The Micra device appears as small battery-shaped electronic device overlying the expected location of the right ventricular apex (Figure 12) on radiography (4).
Device-specific complications
Device complications include cardiac perforation and thromboembolism (33,34).
Leadless pacemaker mimic: implantable loop recorder
Clinical indication
Implantable loop recorders (Medtronic Inc., Minneapolis, MN) are subcutaneously implantable cardiac monitors to continuously record the cardiac rhythm in patients with unexplained palpitations or syncope (35).
Device make and radiographic appearance
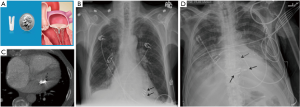
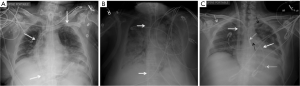
The older device simulates a USB flash-drive, though newer designs are more slender and can be mistaken for a leadless pacemaker on a single frontal-view CXR. The leadless pacemaker has a small dense pointed projection on the tip (Figure 12A,C). The lateral-view CXR clinches the diagnosis as the loop recorder is projected in the subcutaneous chest wall (Figure 13).
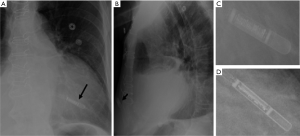
Device specific-complications
Device complications include pain and infection at the site of implantation. Migration is a potential complication.
Ambulatory heart failure monitoring
Clinical indications
Noninvasive measurements of PAP in patients with heart failure can be provided by implantable sensors, which are analogous to those of pulmonary artery catheterization and echocardiography.
Device make and radiographic appearance
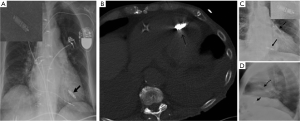
The prototype device named CardioMEMS (St. Jude Medical or Abbott Inc., Chicago, IL) has an inductor coil and a pressure-sensitive capacitor placed in protective housing. It is deployed within a distal pulmonary artery (usually the left lower lobe), and secured in place with nitinol wire loops (Figure 14A,B). The device appears as a small radiopaque line with radiopaque dots on either end and may be missed on portable radiographs (Figure 14C,D). Magnified images and lateral CXRs are often better in depicting the characteristic figure-of-eight shape (Figure 14C, see insert).
Mimic
Without adequate awareness, this may be mistaken for embolized fragmented tip of a vascular catheter or guidewire.
Device-specific complications
Complications related to CardioMEMS include in-situ thrombosis and pulmonary artery injury resulting in hemoptysis (36).
Other monitoring and pacing devices
The Swan-Ganz catheter is used to monitor pulmonary capillary wedge pressure. It is usually inserted via a central vein (usually the right internal jugular) and traverses the right heart chambers with its tip lodged in a distal main pulmonary artery branch (usually the right).
Traditional cardiac pacemakers and defibrillators comprise an external pulse-generator or battery pack and transvenous leads which usually terminate in the right atrial appendage, right ventricle apex and/or coronary sinus. Newer Bundle of His leads positioned towards the interventricular septum are uncommon and maybe mistaken for abnormal placement (Figure 15).
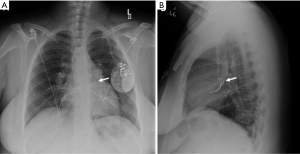
Prevention of thromboembolism in the systemic and pulmonary circulation
Percutaneous left atrial appendage (LAA) closure devices
Clinical indications
LAA closure devices are used in atrial fibrillation as alternatives to anticoagulation or in patients with contraindications to anticoagulation (37).
Device make and radiographic appearance
The Watchman device, Amplatzer Amulet, and Amplatzer Cardiac Plug are prototype LAA closure devices that are placed percutaneously. Watchman device (Atritech/Boston Scientific, Plymouth, MN) has a parachute shape and is often described as appearing jellyfish-like. It is composed of a self-expanding nitinol frame, with proximal face covered by a polyethylene terephthalate fabric (Figure 16A,B). The nitinol frame may be faintly visualized over the LAA location on frontal CXRs, and is often better depicted on lateral CXRs (Figure 16C,D).
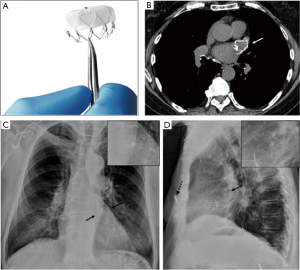
Device-specific complications
Lack of visualization in the expected LAA location on a radiograph may suggest device malposition or migration.
Caval filters
Clinical indication
SVC filters are less common than IVC filters, as incidence of lower extremity deep vein thrombosis (DVT) is way more common. An SVC filter is a safe and effective device for prevention of pulmonary embolism in patients with upper extremity DVT who have contraindication to anticoagulation. Swans and central venous catheters can be placed safely through the filter.
Device make and radiographic appearance of caval filters
A vena cava filter is a small metal device shaped rather like the spokes of an umbrella, which projects over the SVC or IVC with its pointed tip directed towards the right atrium (Figure 17).
Device specific complications of SVC filter
Device migration, fracture, and dislodgement are uncommon potential complications.
Mimic
A deformed SVC stent may mimic SVC filter.
Key features
- TAVR/TAVI and TPVR devices share a common stent-like shape, and project over the expected locations of the aortic and pulmonic valves respectively.
- Mitral and tricuspid clips as the names imply, are short clip-like devices visualized over the mitral or tricuspid valve position (usually two placed closely parallel). Their shape may be mistaken for similar appearing endoscopic clips used for treatment of esophageal achalasia (per-oral endoscopic myotomy clips), though the latter are typically more midline on a frontal CXR (in keeping with the anatomic location of the thoracic esophagus).
- Clamshell-shaped septal occluder devices project over the inter-atrial and ventricular septum.
- A PDA occluder device also has a unique location in the aorto-pulmonary window and may come in one of several designs.
- A stent placed across an aortic coarctation is typically placed in the distal aortic arch/proximal descending aorta.
- The left ventricular partitioning device projects within the left ventricle cavity and has an umbrella or parachute-like design.
- Several catheter-based therapeutic and monitoring devices remain connected with long intravascular catheters or wires which extend outside the chest. These include the Impella, IABP, ECMO, PA catheters and various pacemaker/ICDs.
- Devices that overlie the right ventricular apex include the leadless cardiac pacemaker with the shape of a capsule, which can mimic a loop recorder on a frontal-view CXR.
- Implanted loop-recorders are placed superficially within a subcutaneous pocket of the left anterior chest wall and can be clearly distinguished from the intra-cardiac leadless pacemaker (typically within the RV cavity) on a lateral CXR.
- A distinct, figure-of-eight shaped device which is used for monitoring PAPs in patients with heart failure (CardioMEMS), is usually placed within a distal branch of the left lower lobe pulmonary artery. Without heightened awareness of its presence, this small device may either be missed on the frontal radiograph (given its location and small size) or may simulate embolization of foreign body such as the fragmented tip of a vascular catheter.
- Several commercially available LAA closure devices are available, with the most common being the Watchman device, which has a parachute shape (jellyfish-like design).
- Caval filters in the IVC and SVC are easily identifiable given their characteristic location.
MR compatibility of medical devices and implants
Implanted transcatheter medical devices, particularly active implants or those with ferromagnetic material, can cause harm within the MRI environment. Standard MR safety terminologies include “MR safe,” “MR unsafe,” and “MR conditional”. MR-safe devices are non-hazardous in all MRI environments (non-conducting, non-metallic, and non-magnetic items). MR-unsafe devices are considered to be contraindicated in any MR environments. An MR-conditional device has manufacturer-specified operating conditions to allow MR imaging while minimizing risk such as the main magnetic field strength, maximum magnetic field gradient, and maximum specific absorption rate (SAR). Devices may be safe or conditional, but may impact MR image quality due to artefact (38).
Screening for these devices with CXR is an essential step prior to performing MRI. The MRI safety labeling of the discussed devices is summarized in Table-1. Best practice, whenever there is lack of documentation and certainty regarding a specific device’s MR safety, is to review the device’s information at www.mrisafety.com (30). Many manufacturers provide online MRI safety information and guidelines for their devices. In some cases, the device model numbers are needed to determine the degree of MRI compatibility and a note from the patient’s surgeon may be required to confirm details, though not always feasible (39).
Limitations
An exhaustive list of all cardiothoracic devices is beyond the scope of this pictorial essay. As the title indicates, we have highlighted only the newer segment of the device market that are placed using a trans-catheter approach and excluded all surgically placed intra-cardiac devices as well as aortic stent-graft repairs. We have chosen to omit most of the traditional devices such as central lines, pacemakers and defibrillators, and very briefly discussed intra-aortic balloon pumps and pulmonary artery catheters, as these have long since become mainstream and gained widespread familiarity in the imaging community. Lastly, we decided to leave out a discussion on coronary artery stents as these are much better evaluated using coronary angiography and almost always MRI compatible.
Conclusions
The radiographic findings of contemporary trans-catheter cardiovascular devices discussed, their indications, MRI compatibility, and potential radiographic mimcs are summarized in Table 1. Safety should always be confirmed and documented by reviewing device-specific information (including www.mrisafety.com if needed) prior to patient entry into the MR scanning area.
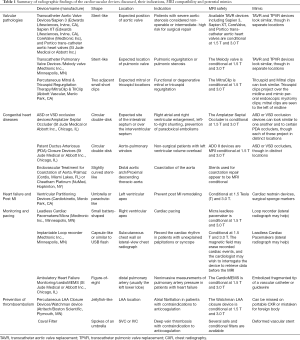
Full table
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-617). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fanari Z, Weintraub WS. Cost-effectiveness of transcatheter versus surgical management of structural heart disease. Cardiovasc Revasc Med 2016;17:44-7. [Crossref] [PubMed]
- Kapadia SR, Leon MB, Makkar RR, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2485-91. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Sigakis CJG, Mathai SK, Suby-Long TD, et al. Radiographic Review of Current Therapeutic and Monitoring Devices in the Chest. Radiographics 2018;38:1027-45. [Crossref] [PubMed]
- Pasic M, Unbehaun A, Buz S, et al. Annular rupture during transcatheter aortic valve replacement: classification, pathophysiology, diagnostics, treatment approaches, and prevention. JACC Cardiovasc Interv 2015;8:1-9. [Crossref] [PubMed]
- Salgado RA, Budde RP, Leiner T, et al. Transcatheter aortic valve replacement: postoperative CT findings of Sapien and CoreValve transcatheter heart valves. Radiographics 2014;34:1517-36. [Crossref] [PubMed]
- Hascoët S, Acar P, Boudjemline Y. Transcatheter pulmonary valvulation: current indications and available devices. Arch Cardiovasc Dis 2014;107:625-34. [Crossref] [PubMed]
- Tretter JT, Friedberg MK, Wald RM, et al. Defining and refining indications for transcatheter pulmonary valve replacement in patients with repaired tetralogy of Fallot: Contributions from anatomical and functional imaging. Int J Cardiol 2016;221:916-25. [Crossref] [PubMed]
- McElhinney DB, Hennesen JT. The Melody® valve and Ensemble® delivery system for transcatheter pulmonary valve replacement. Ann N Y Acad Sci 2013;1291:77-85. [Crossref] [PubMed]
- Saremi F, Gera A, Ho SY, et al. CT and MR imaging of the pulmonary valve. Radiographics 2014;34:51-71. [Crossref] [PubMed]
- Jilaihawi H, Hussaini A, Kar S. MitraClip: a novel percutaneous approach to mitral valve repair. J Zhejiang Univ Sci B 2011;12:633-7. [Crossref] [PubMed]
- Feldman T, Kar S, Rinaldi M, et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol 2009;54:686-94. [Crossref] [PubMed]
- Pighi M, Estevez-Loureiro R, Maisano F, et al. Immediate and 12-Month Outcomes of Ischemic Versus Nonischemic Functional Mitral Regurgitation in Patients Treated With MitraClip (from the 2011 to 2012 Pilot Sentinel Registry of Percutaneous Edge-To-Edge Mitral Valve Repair of the European Society of Cardiology). Am J Cardiol 2017;119:630-7. [Crossref] [PubMed]
- Nickenig G, Weber M, Lurz P, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet 2019;394:2002-11. [Crossref] [PubMed]
- Moore J, Hegde S, El-Said H, et al. Transcatheter device closure of atrial septal defects: a safety review. JACC Cardiovasc Interv 2013;6:433-42. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e143-263. [Crossref] [PubMed]
- Snijder RJ, Suttorp MJ, Berg JM, et al. Percutaneous closure of secundum type atrial septal defects: More than 5-year follow-up. World J Cardiol 2015;7:150-6. [Crossref] [PubMed]
- Sudhakar P, Jose J, George OK. Contemporary outcomes of percutaneous closure of patent ductus arteriosus in adolescents and adults. Indian Heart J 2018;70:308-15. [Crossref] [PubMed]
- Gruenstein DH, Ebeid M, Radtke W, et al. Transcatheter closure of patent ductus arteriosus using the AMPLATZER™ duct occluder II (ADO II). Catheter Cardiovasc Interv 2017;89:1118-28. [Crossref] [PubMed]
- Boudjemline Y. The new Occlutech(®) patent ductus arteriosus occluder: Single centre experience. Arch Cardiovasc Dis 2016;109:384-9. [Crossref] [PubMed]
- Azhar AS, Abd El-Azim AA, Habib HS. Transcatheter closure of patent ductus arteriosus: Evaluating the effect of the learning curve on the outcome. Ann Pediatr Cardiol 2009;2:36-40. [Crossref] [PubMed]
- Forbes TJ, Gowda ST. Intravascular stent therapy for coarctation of the aorta. Methodist Debakey Cardiovasc J 2014;10:82-7. [Crossref] [PubMed]
- Tyagi S, Singh S, Mukhopadhyay S, et al. Self- and balloon-expandable stent implantation for severe native coarctation of aorta in adults. Am Heart J 2003;146:920-8. [Crossref] [PubMed]
- Ledesma M, Alva C, Gómez FD, et al. Results of stenting for aortic coarctation. Am J Cardiol 2001;88:460-2. [Crossref] [PubMed]
- Pilla CB, Fontes VF, Pedra CA. Endovascular stenting for aortic coarctation. Expert Rev Cardiovasc Ther 2005;3:879-90. [Crossref] [PubMed]
- Marshall AC, Perry SB, Keane JF, et al. Early results and medium-term follow-up of stent implantation for mild residual or recurrent aortic coarctation. Am Heart J 2000;139:1054-60. [Crossref] [PubMed]
- Konstam MA, Kramer DG, Patel AR, et al. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging 2011;4:98-108. [Crossref] [PubMed]
- Costa MA, Pencina M, Nikolic S, et al. The PARACHUTE IV trial design and rationale: percutaneous ventricular restoration using the parachute device in patients with ischemic heart failure and dilated left ventricles. Am Heart J 2013;165:531-6. [Crossref] [PubMed]
- Costa MA, Mazzaferri EL Jr, Sievert H, et al. Percutaneous ventricular restoration using the parachute device in patients with ischemic heart failure: three-year outcomes of the PARACHUTE first-in-human study. Circ Heart Fail 2014;7:752-8. [Crossref] [PubMed]
- Shellock FG. MRI Safety. Accessed June 24, 2020. Available online: http://www.mrisafety.com/
- Mohamed I, Lau CT, Bolen MA, et al. Building a bridge to save a failing ventricle: radiologic evaluation of short- and long-term cardiac assist devices. Radiographics 2015;35:327-56. [Crossref] [PubMed]
- Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013;61:e6-75. [Crossref] [PubMed]
- Knops RE, Tjong FV, Neuzil P, et al. Chronic performance of a leadless cardiac pacemaker: 1-year follow-up of the LEADLESS trial. J Am Coll Cardiol 2015;65:1497-504. [Crossref] [PubMed]
- Reynolds DW, Ritter P. A Leadless Intracardiac Transcatheter Pacing System. N Engl J Med. 2016;374:2604-5. [Crossref] [PubMed]
- Aguilera AL, Volokhina YV, Fisher KL. Radiography of cardiac conduction devices: a comprehensive review. Radiographics 2011;31:1669-82. [Crossref] [PubMed]
- Rali AS, Shah Z, Sauer AJ, et al. Hemoptysis After CardioMEMS Implantation: Case Report and Review. Am J Case Rep 2018;19:382-5. [Crossref] [PubMed]
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1-12. [Crossref] [PubMed]
- Shellock FG, Woods TO, Crues JV 3rd. MR labeling information for implants and devices: explanation of terminology. Radiology 2009;253:26-30. [Crossref] [PubMed]
- Tsai LL, Grant AK, Mortele KJ, et al. A Practical Guide to MR Imaging Safety: What Radiologists Need to Know. Radiographics 2015;35:1722-37. [Crossref] [PubMed]


