Lessons from the short- and mid-term outcome of medical rehabilitation in adults with congenital heart disease
Introduction
Congenital malformations of the heart or the great arteries are the most common anomalies in humans, encompassing a great variety of anomalies from simple to complex congenital heart defects (CHD). If left untreated, at least the more complex lesions are often fatal in the first years of life (1). While in the 1940s, most patients with hemodynamically relevant CHD died before reaching adulthood, today up to 95% reach adulthood due to the prodigious diagnostic and therapeutic advances in cardiology, heart surgery, intensive care medicine and pharmacological treatment (2-4).
Although almost all CHD are amenable to treatment, CHD can only be repaired, not cured. Many surgical or interventional procedures result in anatomical and functional residua and sequels that cause health problems later in life and require lifelong dedicated follow-up (5,6). Typical residua and sequels of the specific treatment that negatively influence morbidity and mortality in adulthood encompass heart failure, cardiac arrhythmias, pulmonary vascular disease, aortopathy, endocarditis as well as acquired comorbidities (7-10). Therefore, most adults with congenital heart defects (ACHD) require specific rehabilitation measures, particularly after operations, re-operations, interventional or electrophysiological treatment of serious complications or diseases and after surviving sudden cardiac death.
The ultimate goal of rehabilitation measures in cardiology is to reduce cardiac morbidity and mortality (11,12). It is well known from the study of patients with acquired heart disease that after operations or serious illnesses, targeted cardiological rehabilitation can contribute to regain and maintain the best possible individual physical and mental health as well as social integration in the long term by reducing typical cardiovascular risk factors such as obesity, arterial hypertension, lipid disorders by improving physical performance, providing targeted psycho-cardiological care and social, occupational reintegration (13).
While rehabilitation programs for patients with acquired heart disease have been extensively studied, there are no comprehensive, evidence-based data on rehabilitation programs for ACHD (14). Therefore, the aim of the current study was to obtain first comprehensive data on the specific needs of ACHD requiring medical rehabilitation. Another objective was to develop a concept for the implementation of rehabilitation measures for ACHD on the basis of the acquired experience. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-727).
Methods
This is a retrospective cohort study on the medical care and rehabilitation of ACHD, initiated by the department of Congenital Heart Disease and Paediatric Cardiology of the German Heart Centre Munich in cooperation with the rehabilitation clinic “Klinik Höhenried”.
Inclusion criteria were the presence of a CHD, a completed rehabilitation measure, a medical follow-up and the written consent to participate in the study. Exclusion criteria were the lack of cognitive competence to consent to research and the refusal to consent.
The medical records of the inpatient stay and the follow-up were analyzed. Based on the patient’s medical history and the clinical assessment of the treating physicians, all patients were classified into one of four functional classes (according to PERLOFF) (5). This classification was developed specifically for ACHD and is similar to the NYHA classification of heart failure. Since the symptoms of functional classes I and II as well as III and IV are fluent, they were each grouped into a joint functional class I/II or III/IV for statistical analysis. Furthermore, according to the underlying heart defect, patients were assigned to one of three disease severity classes (simple, moderate, severe) following the recommendations of the American College of Cardiology (15).
The study protocol was reviewed and approved by the ethics committees of the participating institutions (project number: 5338/12). All included patients were informed in detail about the planned study in an explanatory interview and by written information. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients gave their written consent to participate in the study.
Participation or non-participation in this study had no influence on the medical care of the patients.
Data collection and processing were carried out in compliance with the respective federal and state data protection laws. All statistical analyses were made anonymously and not related to individuals.
Statistical analysis
Continuous data were expressed as mean ± standard deviation, median and range. Categorical or interval scaled variables were expressed as absolute numbers or percentages. The data analysis was performed using SPSS 25.0 (IBM Inc., Armonk, NY, USA).
Results
Study sample and patient characteristics
A total of 205 consecutively recruited ACHD, aged ≥18 years, were included in the present study. Two patients, who had not yet reached the numerical age of 18 years, were also classified as “adults” according to their maturity. A total of 110 patients (53.7%) were female. Median patient’s age at the end of their hospital stay at the German Heart Center Munich was 31 years (range: 16–68 years; mean: 33.8±12.3 years).
Main diagnosis and assignment to “ACC Severity Code” Class
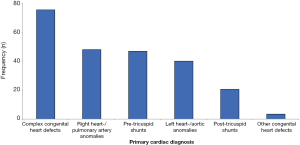
The main cardiac diagnoses were grouped into six categories: (I) complex anomalies, (II) anomalies of the right heart, pulmonary valve or pulmonary artery, (III) anomalies of the left heart, aortic valve or aorta, (IV) pre-tricuspid shunts, (V) post-tricuspid shunts, and; (VI) other anomalies.
Of the 205 included ACHD, 66 (32.2%) had complex heart defects, 42 (20.5%) had right heart/pulmonary abnormalities, 35 (17.1%) had left heart/aortic abnormalities, 41 (20%) had pre-tricuspid shunts, 18 (8.8%) had post-tricuspid shunts and 3 (1.4%) other congenital anomalies (Figure 1).
Ten patients had a syndromic disease associated with their cardiac abnormality: Trisomy-21 (Down syndrome) (n=4), Marfan syndrome (n=3), Microdeletion 22q11 (n=2) and Turner syndrome (n=1).
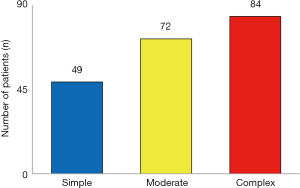
According to the recommendations of the American College of Cardiology, 49 patients (23.9%) could be assigned to the simple, 72 patients (35.1%) to the moderate and 84 patients (41.0%) to the complex CHD-group (Figure 2).
Previous surgical and/or interventional cardiac procedures
The number of procedures performed for each underlying heart defect is given in Table 1. Of all patients, 202 patients (98.5%) had at least one heart operation since birth due to their CHD (Table 2).
Table 1
| Congenital heart defect | Frequency | % | Operation without/with | Intervention without/with | Age, median (range) |
|---|---|---|---|---|---|
| Atrial septal defect | 39 | 19.0 | 0/39 | 29/10 | 31 [18–68] |
| Tetralogy of Fallot | 32 | 15.6 | 0/32 | 26/6 | 31 [17–65] |
| Ebstein’s anomaly | 31 | 15.1 | 1/30 | 24/7 | 40 [19–64] |
| Aortic valve stenosis | 14 | 6.8 | 0/14 | 11/3 | 29 [16–46)] |
| Transposition of the great arteries (TGA) | 13 | 6.3 | 0/13 | 6/6 | 31 [19–45] |
| Coarctation of the aorta | 13 | 6.3 | 0/13 | 12/2 | 31 [21–61] |
| Ventricular septal defect | 11 | 5.4 | 0/11 | 8/3 | 26 [18–68] |
| Aortic valve insufficiency, congenital | 8 | 3.9 | 0/8 | 8/0 | 36 [20–51] |
| Tricuspid atresia | 8 | 3.9 | 1/7 | 4/4 | 34 [29–51] |
| Complete atrioventricular septum defect | 6 | 2.9 | 0/6 | 4/2 | 25 [20–53] |
| Pulmonary atresia with ventricular septal defect | 6 | 2.9 | 0/6 | 3/3 | 26 [21–40] |
| Double inlet ventricle | 5 | 2.4 | 0/5 | 3/2 | 34 [23–43] |
| Congenitally corrected transposition of the great arteries | 3 | 1.5 | 0/3 | 2/1 | [18–52] |
| Double outlet right ventricle (Fallot type) | 2 | 1.0 | 0/2 | 2/0 | 27–22 |
| Double outlet right ventricle (TGA type) | 2 | 1.0 | 0/2 | 1/1 | 24–27 |
| Persistent foramen ovale | 2 | 1.0 | 0/2 | 1/1 | 39–53 |
| Pulmonary atresia | 2 | 1.0 | 0/2 | 1/1 | 21–21 |
| Pulmonary valve stenosis | 2 | 1.0 | 0/2 | 1/1 | 29–65 |
| Truncus arteriosus communis | 2 | 1.0 | 0/2 | 2/0 | 26–44 |
| Aortic aneurysm | 1 | 0.5 | 0/1 | 1/0 | 19 |
| Ductus arteriosus | 1 | 0.5 | 1/0 | 1/0 | 27 |
| Mitral valve insufficiency, congenital | 1 | 0.5 | 0/1 | 1/0 | 24 |
| Mitral valve prolapse, congenital | 1 | 0.5 | 0/1 | 1/0 | 30 |
| Total | 205 | 100 | 3/202 | 152/53 | 31 [16–68] |
Table 2
| Treatment status | Frequency | In % |
|---|---|---|
| No procedure | 3 out of 205 | 1.5 |
| Surgical treatment and/or intervention | 202 out of 205 | 98.5 |
| ● Surgical treatment (≥1) | 202 out of 205 | 98.5 |
| o Re-operation (≥1) | 110 out of 201 | 54.7 |
| ● Interventional treatment (≥1) | 41 out of 205 | 20.0 |
| o Re-intervention (≥1) | 11 out of 41 | 26.8 |
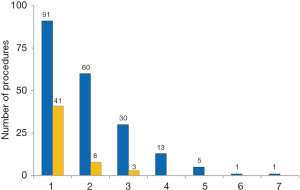
Of the patients operated, 110 (54.7%) were re-operated at least once because of their CHD, while an interventional treatment was performed in 41 patients (20.0%).
Several patients had up to six re-operations and up to two re-interventions (Figure 3).
Interventions performed at the German Heart Center Munich as an indication for rehabilitation measures
Immediately prior to the start of rehabilitation, 189 (92.2%) patients had reparative heart surgery, one patient (0.5%) palliative surgery and six patients (2.9%) an interventional treatment (Table 3).
Table 3
| Surgical treatment | Pre-rehabilitation measures, frequency (n) |
|---|---|
| ASD patch closure | 42 |
| Homograft implantation | 32 |
| Valvuloplasty | 24 |
| Mechanical valve replacement | 19 |
| Biological valve replacement | 17 |
| Conduit/vascular prosthesis | 16 |
| Valvuloplasty - and/or replacement + vessel (Bentall-/ David-OP) | 14 |
| VSD patch closure | 8 |
| Fontan operation | 4 |
| Pacemaker implantation | 4 |
| PFO closure | 2 |
| AVSD patch closure + cleft stitching | 2 |
| Mechanical valve replacement + Homograft | 1 |
| Mechanical valve replacement + VSD patch closure | 1 |
| Other interventions* | 10 |
| Total | 196 |
*, resection of the atrial septum and a membrane at the inferior vena cava (partial anomalous pulmonary vein return), baffle revision and resection of an LVOTO (double outlet right ventricle, aortic coarctation, hypoplasia of the transverse aortic arch and PDA). Mustard-Brom-operation (TGA), coil-occlusion of the internal thoracic artery (pulmonary atresia with ventricular septal defect). Corrective surgery (sinus-venosus defect). Atrioseptostomy and aortopulmonary shunt (double inlet left ventricle), relocation of a left superior vena cava (TGA), total cavopulmonary anastomosis (congenitally corrected transposition), correction of a partial anomalous pulmonary vein return) (sinus-venosus defect), patch closure of aortal wall defects (ASD). ASD, atrial septal defect; VSD, ventricular septal defect; PFO, patent foramen ovale; AVSD, atrioventricular septal defect; LVOTO, left ventricular outflow tract obstruction; PDA, patent ductus arteriosus; TGA, transposition of the great arteries.
In nine patients (4.4%), rehabilitation was not preceded by any surgical or interventional intervention. Of these nine patients, four [double inlet ventricle (n=2); DORV-Fallot type (n=1); tricuspid atresia (n=1)] had relevant arrhythmias requiring cardioversion or electrophysiological treatment.
An electrophysiological treatment for cardiac-arrhythmia was performed in 30 patients before rehabilitation, including pacemaker- (n=29) or ICD-implantation (n=1). Of these, 14 patients had atrial arrhythmias, one had ventricular arrhythmias, two had a second-degree and 13 had a third-degree AV block (Table 4).
Table 4
| Cardiac diagnosis | Type of arrhytmia | Pacemaker | ICD |
|---|---|---|---|
| Aortic valve stenosis | AVB-3 (n=1) | 1 | 0 |
| Atrial septal defect | AVB-3 (n=1) | 1 | 0 |
| Coarctation of the aorta | AVB-2 (n=1); AVB-3 (n=1) | 2 | 0 |
| Complete atrioventricular septal defect | AVB-3 (n=1); SVA (n=1) | 2 | 0 |
| Congenitally corrected transposition of the great arteries | AVB-3 (n=1) | 1 | 0 |
| DORV-TGA | AVB-3 (n=1) | 1 | 0 |
| Double inlet ventricle | SVA (n=1); VA (n=1) | 1 | 1 |
| Ebstein’s anomaly | AVB-3 (n=3); SVA (n=2) | 5 | 0 |
| Persistent Foramen Ovale | SVA (n=1) | 1 | 0 |
| Pulmonary atresia + VSD | SVA (n=1) | 1 | 0 |
| Tetralogy of Fallot | SVA (n=2) | 2 | 0 |
| Transposition of the great arteries | AVB-3 (n=3); SVA (n=2) | 5 | 0 |
| Tricuspid atresia | SVA (n=4) | 4 | 0 |
| Ventricular septal defect | AVB-2 (n=1); AVB-3 (n=1) | 2 | 0 |
| Total | 29 | 1 |
AVB, AV-Block 2nd or 3rd degree; SVA, supraventricular arrhythmias; VA, ventricular arrhythmias; DORV-TGA, double outlet right ventricle - transposition of the great arteries type; VSD, ventricular septal defect.
Clinical data prior to the transfer from the German Heart Center to the Rehabilitation Clinic and Course of Rehabilitation
At the time of referral to the rehabilitation clinic, immediately after the end of hospital treatment, all patients were fully compensated and without signs of heart failure. Out of 205 patients, 189 (92.2%) were in PERLOFF Functional class I/II and 16 (7.8%) in Functional class III/IV, although 156 (n=76.1%) were classified as moderate or complex CHD, according to the ACC severity code, and 15 (7.3%) suffered from Eisenmenger syndrome or chronic cyanosis.
During the entire rehabilitation, no critical cardiac events occurred. None of the 30 patients with relevant arrhythmias, with a history of thromboembolic events (n=12) or infectious endocarditis (n=5) had a relapse during the rehabilitation period.
After a mean rehabilitation duration of 24 days (median: 21 days; range: 9–50 days) the number of patients in Functional class I/II increased from 189 to 200, with only five patients (Ebstein’s anomaly; pulmonary atresia with ventricular septal defect; pulmonary valve stenosis; tricuspid atresia; double inlet ventricle) remaining in Functional class III/IV (Table 5).
Table 5
| Cardiac diagnosis | Before | After | |
|---|---|---|---|
| I/II versus III/IV | I/II versus III/IV | ||
| Atrial septal defect | 38 // 1 | 39 // 0 | |
| Tetralogy of Fallot | 31 // 1 | 32 // 0 | |
| Ebstein’s anomaly | 27 // 4 | 30 // 1 | |
| Coarctation of the aorta | 12 // 1 | 13 // 0 | |
| Transposition of the great arterias | 12 // 1 | 13 // 0 | |
| Tricuspid atresia | 5 // 3 | 7 // 1 | |
| Pulmonary atresia with ventricular septal defect | 5 // 1 | 5 // 1 | |
| Double inlet ventricle | 4 // 1 | 4 // 1 | |
| Pulmonary valve stenosis | 0 // 2 | 1 // 1 | |
| Persistent ductus arteriosus | 0 // 1 | 1 // 0 | |
| Other diagnosis* | 55 // 0 | 55 // 0 | |
| Total | 189 // 16 | 200 // 5 |
*, aortic valve stenosis, ventricular septal defect, aortic valve insufficiency, complete AV septal defect, congenitally corrected transposition of the great arteries, pulmonary atresia, double outlet right ventricle (TGA type), truncus arteriosus communis, double outlet right ventricle (Fallot type), persistent foramen ovale, mitral valve insufficiency, mitral valve prolapse, aortic aneurysm.
At the time of transfer to the rehabilitation clinic, 95% of the patients were on chronic medication. After rehabilitation, the number of patients receiving diuretics, beta-blockers, amiodarone, digitalis glycosides, AT-blockers, Ca-Antagonists or oral anticoagulants decreased, while during the rehabilitation the prescription of all drugs increased (Table 6).
Table 6
| Medication | During hospital stay | At the end of rehabilitation | After rehabilitation | ||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| Currently no medication | 11 [5] | 6 [3] | 37 [18] | ||
| Primary cardiac drugs | |||||
| ● Diuretics | 135 [66] | 148 [72] | 77 [38] | ||
| ● ß-Blockers | 71 [35] | 148 [72] | 90 [44] | ||
| ● ACE inhibitors | 21 [10] | 27 [13] | 28 [14] | ||
| ● Amiodarone | 12 [6] | 20 [10] | 10 [5] | ||
| ● Digitalis glycosides | 8 [4] | 13 [6] | 11 [5] | ||
| ● Antiarrhythmics, other | 5 (2.5) | 3 (1.5) | 3 (1.5) | ||
| ● AT-Blocker | 4 [2] | 8 [4] | 5 (2.5) | ||
| ● Ca-Antagonists | 2 [1] | 3 (1.5) | 2 [1] | ||
| Anticoagulants | |||||
| ● Anticoagulants, oral | 74 [36] | 106 [52] | 65 [32] | ||
| ● Platelet inhibitors | 12 [6] | 10 [5] | 10 [5] | ||
| Primary non-cardiac drugs | |||||
| ● Thyreoid hormones | 29 [14] | 39 [19] | 41 [20] | ||
| ● Antihyperlipidemic agents | 5 (2.5) | 7 (3.5) | 8 [4] | ||
| ● other* | 94 [46] | 122 [60] | 85 [41] | ||
| No information | 39 [19] | 0 | 8 [4] | ||
*, others: Proton pump inhibitors, Antihistamines, Antiepileptics, Allopurinol, Antidepressants, Contraceptives, Analgetics, Antibiotics, Benzodiazepine, Glucocorticoids, Minerals/Vitamins. ACE, angiotensin-converting-enzyme; AT, angiotensin; Ca, calcium.
While prior to the medical treatment 157 patients were capable of working, the number increased to 168 after rehabilitation. Only 6 patients (2.9%) [Ebstein’s anomaly (n=3), Ductus arteriosus (n=1), tetralogy of Fallot (n=1) and aortic valve regurgitation (n=1)] were discharged as “unable to work”. This is equivalent to the 4% of patients who were actively working/job-seeking before the cardiac intervention.
Mid-term outcome after completion of the rehabilitation
Between discharge from rehabilitation and follow-up at the German Heart Center Munich (mean time interval 28 months, median: 22 months; range: 0–172 months), the number of patients in Functional class I/II decreased from 200 to 194, while the number in Functional class III/IV increased from 5 to 11 patients, including patients with atrial septal defect (n=1), tetralogy of Fallot (n=1), tricuspid atresia (n=1), Ebstein’s anomaly (n=3), pulmonary atresia with ventricular septal defect (n=1), double inlet ventricle (n=1) and Ductus arteriosus (n=1). Cardiac decompensation occurred in four patients (2%) with Ebstein’s anomaly, tetralogy of Fallot, complete transposition or atrial septal defect.
Treatment-relevant atrial arrhythmias (n=5) or a third-degree AV block (n=1) occurred in patients with atrial septal defect (n=1), aortic valve stenosis (n=1), complete transposition (n=1), tricuspid atresia (n=1), or tetralogy of Fallot (n=2).
Between discharge from rehabilitation and the follow-up visit no patient was diagnosed with an infectious endocarditis. One 26-year-old woman with tricuspid atresia developed a thromboembolic event.
All in all, during follow-up, 19 patients (9.3%) needed further cardiac interventions (Table 7). One patient with persistent Ductus arteriosus was listed for lung transplantation and another patient (Ebstein’s anomaly) refused recommended corrective surgery.
Table 7
| Cardiac diagnosis | Sex, m/f | Age (years) | Interventions after rehabilitative measures |
|---|---|---|---|
| Aortic valve stenosis | M | 16 | Pacemaker implantation |
| Atrial septal defect | F | 61 | Pacemaker implantation |
| Double outlet right ventricle (Fallot type) | F | 27 | Stent implantation in RVOT |
| DORV-TGA | M | 27 | Additional RV-PM probe implantation |
| Ebstein’s anomaly | F | 32 | Pacemaker implantation |
| Ebstein’s anomaly | F | 19 | Amplatzer-ASD-Occluder |
| Pulmonary atresia + VSD | M | 21 | VSD closure |
| Pulmonary valve stenosis | F | 29 | PDA closure |
| Tetralogy of Fallot | M | 65 | Coronary stent |
| Tetralogy of Fallot | F | 65 | Pacemaker implantation |
| Tetralogy of Fallot | M | 19 | Stent implantation in RV-PA-Homograft and Melody valve |
| Tetralogy of Fallot | F | 36 | Pacemaker implantation |
| Tetralogy of Fallot | M | 30 | Stent implantation and implantation of a Melody valve |
| Transposition of the great arteries | F | 34 | ICD implantation |
| Transposition of the great arteries | M | 36 | MV replacement, TV-plastic, MV exchange, pacemaker revision |
| Transposition of the great arteries | M | 29 | Tricuspid valve occlusion occlusion |
| Tricuspid atresia | F | 36 | Coil closure of veno-venous collaterals |
| Tricuspid atresia | M | 43 | Pacemaker implantation |
| Truncus arteriosus communis | M | 26 | Balloon dilatation of the pulmonary artery + stent implantation (pulmonary artery) |
M, male; F, female; RVOT, right ventricular outflow tract; DORV-TGA, double outlet right ventricle - transposition of the great arteries type; RV, right ventricle; PM, pacemaker; ASD, atrial septal defect; VSD, ventricular septal defect; PDA, patent ductus arteriosus; PA, pulmonary artery; ICD, implantable cardioverter-defibrillator; MV, mitral valve; TV, tricuspid valve.
Out of the six patients, who were “unable to work” at the end of the rehabilitation measures, three [Ebstein’s anomaly (n=2), aortic valve insufficiency (n=1)] returned to work again and one patient retired.
Discussion
For the first time, the present study provides comprehensive data on the medical rehabilitation of ACHD after previous treatment in a tertiary care center.
The reason for conducting the present study was that despite the increasing number of ACHD worldwide, there are only few structured cardiological rehabilitation programs and few scientific studies on this specific patient population. In contrast, rehabilitation measures for acquired heart disease have been scientifically better investigated. The currently available data on ACHD derive mostly from small, uncontrolled studies with very different types of CHD, due to the fact that until a few decades ago the need for specific rehabilitation of patients with CHD was underestimated (16).
Current study data show that rehabilitation of ACHD requires consideration of special features that differ considerably from those of acquired heart disease. The fact that ACHD often cannot be treated in the same way as acquired heart disease places tremendous demands on rehabilitation facilities.
The data from a large cohort of native, surgically or interventional treated CHD (n=205), which included almost all types and severity degrees [moderate: 35.1% (n=72); severe: 41.0% (n=84)] of CHD and syndromes, showed that many of these patients required medical rehabilitation. Almost all patients (98.6%; n=202) had received up to six re-operations or an interventional treatment (26.8%; n=41) because of their CHD. The age of the included patients was low (median 31 years; range: 16 and 68 years) compared to the age of patients who normally receive rehabilitation due to acquired heart disease.
Although the majority of CHDs were “repaired” in the current study, almost all patients exhibited anatomical and/or functional conditions, residuals and sequelae. These conditions are typical for the pathological anatomy of the CHD or the type of therapeutic intervention and may have a negative impact on the quality of life, performance, work capacity and longevity. This situation also reflects the future requirement of modern rehabilitation facilities. Expectations placed on physicians and nursing staff of a rehabilitation clinic will be increasing in order to provide highly-qualified medical care.
One reason for the rising demand is the fact that the duration of the hospital stays of ACHD after cardiac operations or interventions or cardiac decompensation is becoming shorter and shorter. In addition, post-operative/interventional complications frequently occur in the first weeks and months after a hospital stay, i.e., during the period of rehabilitation. Therefore, physicians and nurses in rehabilitation facilities must be familiar with CHD and technically knowledgeable about how to manage specific complications.
Since CHD patients’ rehabilitation needs are often complex, rehabilitation should preferably be performed in an experienced facility with an ACHD-certified (pediatric) cardiologist and in close cooperation with a supra-regional ACHD-center that can also provide multidisciplinary treatment of non-cardiac ACHD problems (6,17,18).
The use of rehabilitation facilities is further complicated by the fact that therapy goals for successful rehabilitation are currently not sufficiently defined, only cursory guidelines or recommendations exist, and there are no concrete instructions that cover the needs of the ACHD (18). In order to meet the needs of this specific patient population and to take the particularities of symptoms, residuals, complications and problems into account, the near-term development of a structure for the practical implementation of rehabilitation in ACHD is necessary.
The ultimate goal of these efforts is to provide better care for ACHD, maintain or improve their condition and performance through an optimized rehabilitation program and to effect reduced morbidity and mortality. Particular attention should be paid to similarities with and differences from established rehabilitation concepts in acquired cardiovascular diseases (e.g., coronary heart disease, acquired valve changes or cardiac arrhythmias).
All rehabilitation measures, which are partly identical to tertiary prevention, share the commonality that they are intended to alleviate the consequences of the disease, prevent a recurrence, and prevent the disease from worsening. Various measures are applied, which primarily aim to inform patients, educate them, and improve their physical performance.
Acute- and post-acute phase
In the rehabilitation of ACHD, a distinction between the acute and the post-acute phase has to be made. In the acute phase, as in acquired heart disease, a comprehensive initial diagnosis should be made, which takes into account the patient’s medical history, examination findings, previous medical reports and medical findings, resting ECG, echocardiography, exercise ECG and laboratory findings. On the basis of this diagnosis, rehabilitation goals must be set and therapeutic measures prescribed. All therapeutic goals are worked out together with the patient and focus on risk stratification, performance assessment and identification of individual problems (13,19).
In the early rehabilitation period of ACHD, the detection of periprocedural (postoperative/postinterventional) conditions and complications, typical for the specific CHD, is of particular importance. From the patients included in this study it can be deduced that, in particular, ventricular dysfunction, heart failure, pulmonary hypertension or pulmonary vascular disease, cardiac arrhythmias, valve thromboses, thromboembolism, wound healing disorders, fever, post-thoracotomy syndromes, thoracic scaffold pain, anaemia (after intraoperative blood loss, haemolysis), neurological and neurocognitive deficits, and reactive depression or a psychosyndromes are relevant (20,21).
For the management of these patients, it is important to consider the particularities of CHD. In contrast to acquired heart disease, ventricular dysfunction and heart failure in ACHD often affect the right (subpulmonary) ventricle or a morphologically right systemic ventricle. The assessment and treatment of impaired hemodynamics depends largely on the type of CHD and the extent of a ventricular dysfunction which often already exists preoperatively.
As the current study data show, this condition particularly affects complex CHD with chronic pressure or volume stress, e.g., univentricular hearts (after Fontan surgery), transposition of the great vessels after atrial switch operation with a systemic right ventricle, as well as pulmonary vascular disease resulting from a primary left-right shunt or severe heart valve disease, e.g., after repair of a Tetralogy of Fallot (22-27). These patients often respond differently to therapeutic measures than patients with acquired heart disease do (25,27).
In these patients, it is often difficult to detect heart failure using standard or threshold values (e.g., echocardiography), as these may be specific for the type of CHD and may differ from the values for acquired heart disease (e.g., Fontan circulation, after atrial redirection or in congenitally corrected transposition).
In principle, the drugs commonly used in adult cardiology are also used for ACHD, although the specific characteristics of CHD must be taken into account (26-31). Nevertheless, there are many uncertainties regarding therapeutic measures since comprehensive data from controlled studies are lacking (32).
As the study also shows, cardiac arrhythmias, especially in complex CHD, often occur as atrial arrhythmias and less frequently as ventricular arrhythmias. Particularly susceptible are native or pre-operated patients with atrial septal defect, tetralogy of Fallot, transposition of the great arteries after atrial redirection, functionally univentricular heart after Fontan surgery or Ebstein’s anomaly (33). In rehabilitation, the detection and treatment of early postoperative cardiac arrhythmias is, therefore, of great importance (4,34). Additionally, it should be known that supraventricular tachycardias are poorly tolerated and can cause decompensation or death, especially in complex CHD (e.g., after atrial inversion, in Fontan circulation, in cyanotic heart defects, in Eisenmenger’s syndrome) (27).
The secondary goal of antiarrhythmic therapy is to reduce morbidity, maintain ventricular function and improve prognosis by preventing sudden cardiac death. Risk stratification for sudden cardiac death is difficult in CHD and must be highly individualized. Rehabilitation measures should already set the course in the right direction, although the treatment of complex CHD in particular should be reserved for specialists due to the special features typical of the underlying CHD (33-35).
Pulmonary arterial hypertension in ACHD particularly affects shunt lesions (with the extreme form of Eisenmenger’s syndrome), congenital obstructions of the left heart, cyanotic heart disease with increased pulmonary flow, anomalies of the pulmonary artery and, more recently, patients with univentricular heart and Fontan circulation who develop pulmonary vascular disease (36-40).
Also in these patients, the therapy performed has to take the peculiarities of the CHD into account. The use of specific measures and highly effective, but not harmless drugs for pulmonary hypertension treatment, should be coordinated by specialists (26,27,36,39,41) This coordination is particularly true for patients with Eisenmenger’s syndrome (42). Supportive therapy measures include treatment with diuretics, oxygen, oral anticoagulants, phlebotomies and compensation for anemia and/or iron deficiency (41,43). Drugs with systemic vasodilation (e.g., AT blockers, ACE inhibitors) are only applicable in special circumstances, but may also be contraindicated if they amplify an existing right-left shunt and a consecutive deepening of cyanosis. Of particular importance is the specific pharmacotherapy with endothelin antagonists, PDE-5 inhibitors, sGC stimulators, prostanoids, or IP prostacyclin receptor agonists which can significantly improve quality of life and prognosis (39).
In ACHD, special attention must be paid after a recently performed surgery. It is important to note that anaemia in vitally-associated cyanosis is defined, weighted and treated differently than in acquired heart disease (43).
Other common early postoperative/interventional problems concern the detection, evaluation and treatment of newly-occurring or persistent fever and inflammatory reactions (leukocytosis, increase of CRP, SPA, pro-calcitonin, etc.), as seen, for example, in endocarditis early after valve replacement or in wound infections and wound healing disorders. In principle, however, the diagnosis and treatment do not differ significantly from the postoperative procedures for acquired heart defects (44).
For endocarditis prophylaxis, the physician in the rehabilitation clinic must be aware of the special features of ACHD since the incidence of infective endocarditis is higher than in the general population (45,46). Since the mortality rate of the disease is still high and patients are at risk of developing infective endocarditis during rehabilitation, the rehabilitation clinic should be informed about typical symptoms (e.g., fever, night sweats, unclear weight loss, changed findings during auscultation, newly occurring heart failure) and patients should be advised about the necessity and practical implementation of endocarditis prophylaxis.
Rehabilitation in the post-acute phase
In ACHD, advice in the post-acute phase should include somatic, educational, psychological and social areas. The somatic area also includes the agreement of rehabilitation goals and the prescription of therapeutic measures (13,19).
Education should include health promotion and patients should be instructed about the importance of a healthy lifestyle. This lifestyle instruction includes the elimination of nicotine, the acquisition and adherence to a healthy diet, targeted necessary weight reduction and physical exercise. In addition, rehabilitative training is provided as required, e.g., on heart disease, endocarditis prophylaxis and, if necessary, self-management of oral anticoagulation.
Psychosocial support
The current study has shown that the somatic recovery and professional-/social integration of the often quite young patients depends largely on psychological and social factors. ACHD should be supported in the social field, in their professional reintegration as well as in their private environment (e.g., by drawing up a step-by-step reintegration plan and by initiating benefits for participation in working life) (47).
Mental stress often exerts a negative influence on the course of the illness. In some cases, neurological and neurocognitive deficits as well as reactive depression or psychosyndromes have existed since childhood (48). For this reason, screenings for post-traumatic stress disorder, depression, anxiety or social isolation should be included in the diagnostics at the beginning of rehabilitation measures by means of questionnaires and, if necessary, supplemented by individual interviews (13,49,50).
Physical exercise and resilience in ACHD
ACHD themselves often have limited knowledge about their heart defects, especially about their physical capacity and the purpose/implementation of physical training (51).
Although it is known from patients with acquired heart disease and chronic heart failure that adapted aerobic endurance training can improve physical performance, reduce symptoms, improve quality of life and probably reduce morbidity and mortality, there is no reliable data for ACHD. Only small studies show that adapted, aerobic physical endurance training can improve physical performance and thus quality of life in ACHD through improved neurohumoral mechanisms and the adaptation of peripheral circulation. For this reason, rehabilitation measures should include a proposal for a specific training protocol after examining the patient’s individual stress profile, which can and should be continued at home after discharge in order to improve resilience, well-being and other positive psychological changes (52).
In addition to the purely medical issues, a particular need exists for advice on disability and insurance law, old-age provision, forms of education (school, university, profession), earning capacity, obtaining a driving license, airworthiness and often also on pregnancy and inheritance of heart defects.
Health maintenance and prevention
Acquired co-morbidities, which occur frequently and have a lasting and unfavorable effect on the natural course of a CHD, are of particular relevance within a rehabilitation program for ACHD (53-55). In addition to acquired cardiovascular diseases (arterial hypertension, coronary heart disease, valvular diseases, endocarditis), this occurrence concerns the involvement of other organ systems, especially the lungs, pulmonary vessels, kidney, blood, coagulation system, central nervous system and metabolic disorders (diabetes mellitus, hyperlipidemia, hyperuricemia). Since ACHD are reaching an increasingly higher age, education about prevention of aggravation and complications as well as health promotion is paramount.
Final examination
The final examination includes a review of the drug therapy and the intended therapeutic goals, the preparation of an individual training plan for the time after rehabilitation, recommendations for further treatment (including medical check-ups, laboratory values, echocardiography) and the prescription of follow-up and preventive measures (e.g., in the outpatient coronary-sport groups, physiotherapy). In addition, the socio-medical classification is discussed with the patient (including restrictions on benefits and remaining positive performance) (49).
Limitations
Among the strengths of the current study are the large sample size of the patients and the inclusion of all types and severity degrees of CHD. This study has limitations as the study design was retrospective, therefore, no control group exists. The study was conducted at a tertiary center for adults with CHD and at a rehabilitation clinic specialized in ACHD. As a result, the distribution of patients in terms of type and severity of the underlying heart defect does not correspond to the typical patient population as seen by a general practitioner, internist or cardiologist. The prevalence of complex abnormalities is probably much higher in these facilities than in regional hospitals or in regular cardiology departments. The data presented is derived solely from patients living in Germany. The generalization of the conclusions and the transfer to patients living in other countries or different ethnic groups is debatable. Further studies are also required in this respect.
Conclusions
With the primary goal of reducing cardiac morbidity and mortality, cardiac rehabilitation and prevention programs for ACHD are an essential part of health care. Rehabilitation and prevention measures should include the reduction of typical and known cardiovascular risk factors as well as social and occupational reintegration. However, the difference between congenital and acquired heart disease must always be considered and not all measures applied to acquired heart disease can be transferred to ACHD.
The existing, rather modest data on rehabilitation and prevention in congenital heart disease need to be expanded. Randomized controlled studies on rehabilitation measures are necessary to prove the efficiency of follow-up treatment and to develop concepts specific to CHD.
Acknowledgments
The authors thank the German Heart Foundation (“Deutsche Herzstiftung e.V.”), the German patient organization “Herzkind e. V.”, and also the German health care insurance AOK-Bayern for the promotion of ACHD research.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part IV”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-727
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-727
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-727). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part IV” was commissioned by the editorial office without any funding or sponsorship. YVK serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from February 2018 to January 2022. HK serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from February 2018 to January 2022. YVK and HK served as the unpaid Guest Editors of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was reviewed and approved by the ethics committees of the participating institutions (project number: 5338/12). All included patients were informed in detail about the planned study in an explanatory interview and by written information. All patients gave their written consent to participate in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Allen D, Gutgesell H, Clark E. Moss and Adams. Heart Disease in Infants, Children, and Adolescents Including the Fetus and Young Adults. Philadelphia: Wolters Kluwer, 2001.
- Samánek M. Children with congenital heart disease: probability of natural survival. Pediatr Cardiol 1992;13:152-8. [PubMed]
- Webb GD. Care of adults with congenital heart disease--a challenge for the new millennium. Thorac Cardiovasc Surg 2001;49:30-4. [Crossref] [PubMed]
- Warnes CA. The adult with congenital heart disease: born to be bad? J Am Coll Cardiol 2005;46:1-8. [Crossref] [PubMed]
- Perloff J, Child J, Aboulhosn J. Congenital heart disease in adults. Philadelphia: W. B. Saunders Company, 2009.
- Kaemmerer H, Breithardt G. Recommendations for the quality improvement of interdisciplinary care of adults with congenital heart anomalies. Clin Res Cardiol 2006;95:76-84. [Crossref] [PubMed]
- Bjarnason-Wehrens B, Dordel S, Sreeram N, et al. Cardiac Rehabilitation in Congenital Heart Disease. In: Perk J, Gohlke H, Hellemans I, et al. editors. Cardiovascular Prevention and Rehabilitation. London: Springer, 2007.
- Seidel L, Nebel K, Achenbach S, et al. Facts about the General Medical Care of Adults with Congenital Heart Defects: Experience of a Tertiary Care Centre. J Clin Med 2020;9:1943. [Crossref] [PubMed]
- Neidenbach R, Niwa K, Oto O, et al. Improving medical care and prevention in adults with congenital heart disease-reflections on a global problem-part I: development of congenital cardiology, epidemiology, clinical aspects, heart failure, cardiac arrhythmia. Cardiovasc Diagn Ther 2018;8:705-15. [Crossref] [PubMed]
- Neidenbach R, Niwa K, Oto O, et al. Improving medical care and prevention in adults with congenital heart disease - reflections on a global problem-part II: infective endocarditis, pulmonary arterial hypertension and aortopathy. Cardiovasc Diagn Ther 2018;8:716-24. [Crossref] [PubMed]
- Ades PA, Coello C. Effects of exercise and cardiac rehabilitation on cardiovascular outcomes. Med Clin North Am 2000;84:251-65. [Crossref] [PubMed]
- Lavie CJ, Milani R. Benefits of cardiac rehabilitation and exercise training. Chest 2000;117:5-7. [Crossref] [PubMed]
- Bjarnason-Wehrens B, Klaus H, Hoberg E, et al. Deutsche Leitlinie zur Rehabilitation von Patienten mit Herz Kreislauferkrankungen (DLL-KardReha). Clin Res Cardiol 2007;Suppl 2:III/1–III/54.
- Tikkanen AU, Oyaga A, Riano O, et al. Paediatric cardiac rehabilitation in congenital heart disease: a systematic review. Cardiol Young 2012;22:241-50. [Crossref] [PubMed]
- Warnes CA, Liberthson R, Danielson G, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol 2001;37:1170-5. [Crossref] [PubMed]
- Holloway TM, Chesssex C, Grace S, et al. A call for adult congenital heart disease patient participation in cardiac rehabilitation. Int J Cardiol 2011;150:345-6. [Crossref] [PubMed]
- Hess J, Bauer U, de Haan F, et al. Empfehlungen für Erwachsenen- und Kinderkardiologen zum Erwerb der Zusatz-Qualifikation „Erwachsene mit angeborenen Herzfehlern“ (EMAH). Clin Res Cardiol 2007;19-26.
- DGPR, Deutsche Gesellschaft für Prävention und Rehabilitation von Herz-Kreislauferkrankungen. S3-Leitlinie zur kardiologischen Rehabilitation (LL-KardReha) im deutschsprachigen Raum Europas Deutschland, Österreich, Schweiz (D-A-CH), 2020.
- Hoberg E. Allgemeine Gesichtspunkte zur Diagnostik und Überwachung. In Rauch B, Middeke M, Bönner G, Karoff M, Held K (eds.). Kardiologische Rehabilitation- Standards für die Praxis nach den Leitlinien der Deutschen Gesellschaft für Prävention und Rehabilitation von Herz- Kreislauferkrankungen e.V. (DGPR). Stuttgart: Georg Thieme Verlag, 2007.
- Perloff JK, Warnes C. Challenges posed by adults with repaired congenital heart disease. Circulation 2001;103:2637-43. [Crossref] [PubMed]
- Ministeri M, Alonso-Gonzalez R, Swan L, et al. Common long-term complications of adult congenital heart disease: avoid falling in a H.E.A.P. Expert Rev Cardiovasc Ther 2016;14:445-62. [Crossref] [PubMed]
- Engelings CC, Helm H, Abdul-Khaliq B, et al. Cause of death in adults with congenital heart disease - An analysis of the German National Register for Congenital Heart Defects. Int J Cardiol 2016;211:31-6. [Crossref] [PubMed]
- Zomer AC, Uiterwaal C, van der Velde E, et al. Mortality in adult congenital heart disease: are national registries reliable for cause of death? Int J Cardiol 2011;152:212-7. [Crossref] [PubMed]
- Verheugt CL, Uiterwaal C, van der Velde E, et al. Mortality in adult congenital heart disease. Eur Heart J 2010;31:1220-9. [Crossref] [PubMed]
- Piran S, Veldtman G, Siu S, et al. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation 2002;105:1189-94. [Crossref] [PubMed]
- Neidenbach R, Kaemmerer H. Striking Supply Gap in Adults with Congenital Heart Disease? Dtsch Med Wochenschr 2017;142:301-3. [PubMed]
- Neidenbach R, Schelling J, Pieper L, et al. Sind Erwachsene mit angeborenen herzfehlern ausreichend versorgt? Zeitschrift für Herz-, Thorax- und Gefäßchrirurgie 2017;31:228-40.
- Ponikowski P, Voors A, Anker S, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Vamos M, Erath J, Hohnloser S. Digoxin-associated mortality: a systematic review and meta-analysis of the literature. Eur Heart J 2015;36:1831-8. [Crossref] [PubMed]
- Ziff OJ, Kotecha D. Digoxin: The good and the bad. Trends Cardiovasc Med 2016;26:585-95. [Crossref] [PubMed]
- Lindenfeld J, Keller K, Campbell D, et al. Improved systemic ventricular function after carvedilol administration in a patient with congenitally corrected transposition of the great arteries. J Heart Lung Transplant 2003;22:198-201. [Crossref] [PubMed]
- Budts W. Physical activity in adolescents and adults with congenital heart defects: individualized exercise prescription. Eur Heart J 2013;34:3669-74. [Crossref] [PubMed]
- Khairy P, Van Hare G, Balaji S, et al. PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Can J Cardiol 2014;30:e1-e63. [Crossref] [PubMed]
- Oechslin EN, Harrison D, Connelly M, et al. Mode of death in adults with congenital heart disease. Am J Cardiol 2000;86:1111-6. [Crossref] [PubMed]
- Nieminen HP, Jokinen E, Sairanen H. Causes of late deaths after pediatric cardiac surgery: a population-based study. J Am Coll Cardiol 2007;50:1263-71. [Crossref] [PubMed]
- Kaemmerer H, Apitz C, Brockmeier K, et al. Pulmonary hypertension in grown-ups with congenital heart disease: Recommendations of the Cologne Consensus Conference 2016. Dtsch Med Wochenschr 2016;141:S70-9. [PubMed]
- Diller GP, Gatzoulis M. Pulmonary vascular disease in adults with congenital heart disease. Circulation 2007;115:1039-50. [Crossref] [PubMed]
- Duffels MG, Engelfriet PM, Berger RM, et al. Pulmonary arterial hypertension in congenital heart disease: an epidemiologic perspective from a Dutch registry. Int J Cardiol 2007;120:198-204. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery J, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Zimmermann R, Schranz D, Ewert P, et al. Pulmonary arterial hypertension in congenital heart defects with shunt: a heterogeneous and complex constellation. Dtsch Med Wochenschr 2013;138:1244-6. [PubMed]
- Kaemmerer H, Niwa K, Oechslin E, et al. Pulmonary Arterial Hypertension in Congenital Heart Disease: Eisenmenger’s Syndrome – A Global Perspective. Bremen: UNI-MED-Verlag, 2013.
- D'Alto M, Diller G. Pulmonary hypertension in adults with congenital heart disease and Eisenmenger syndrome: current advanced management strategies. Heart 2014;100:1322-8. [Crossref] [PubMed]
- Kaemmerer H, Mebus S, Schulze-Neick I, et al. The adult patient with eisenmenger syndrome: a medical update after dana point part I: epidemiology, clinical aspects and diagnostic options. Curr Cardiol Rev 2010;6:343-55. [Crossref] [PubMed]
- Wilson W, Taubert K, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007;116:1736-54. [Crossref] [PubMed]
- Berglund E, Johansson B, Dellborg M, et al. High incidence of infective endocarditis in adults with congenital ventricular septal defect. Heart 2016;102:1835-39. [Crossref] [PubMed]
- Horstkotte D, Follath F, Gutschik E, et al. Guidelines on prevention, diagnosis and treatment of infective endocarditis executive summary; the task force on infective endocarditis of the European society of cardiology. Eur Heart J 2004;25:267-76. [Crossref] [PubMed]
- Karoff M, Roseler S, Lorenz C, et al. Intensified after-care--a method for improving occupational reintegration after myocardial infarct and/or bypass operation. Z Kardiol 2000;89:423-33. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e143-e263. [Crossref] [PubMed]
- Karoff M, Kittel J. Allgemeiner Aufbau und Inhalte der kardiologische Rehabilitation. In Rauch B, Middeke M, Bönner G, et al. editors. Kardiologische Rehabilitation- Standards für die Praxis nach den Leitlinien der Deutschen Gesellschaft für Prävention und Rehabilitation von Herz- Kreislauferkrankungen e.V. (DGPR). Stuttgart: Georg Thieme Verlag, 2007.
- DGPR. Deutsche Gesellschaft für Prävention und Rehabilitation von Herz-Kreislauferkrankungen. Empfehlungen zu Standards der Prozessqualität in der kardiologischen Rehabilitation (Teil 4). Z Kardiol 2002;91:99-102.
- Neidenbach R, Pieper L, Schelling J, et al. Die Versorgungssituation von Erwachsenen mit angeborenen Herzfehlern (EMAH) aus Sicht der Allgemein Ärzte und Internisten, sowie praktischer Ärzte. Thorac Cardiovasc Surgeon 2017;65:S111-42. [Crossref]
- Gabriel H. Sport bei Patienten mit angeborenen Herzfehlern. Available online: https://www.kup.at/kup/pdf/5240.pdf
- Lummert E, Hauser M, Vigl M, et al. Noncardiac comorbidities of congenital heart disease in adults. Am J Cardiol 2014;7:S109. [Crossref]
- Hauser M, Lummert E, BraunS, et al. Nichtkardiale Komorbiditäten bei erwachsenen Patienten mit angeborenen Herzfehlern. Zeitschrift für Herz-, Thorax- und Gefäßchirurgie 2016; 31:130-7.
- Tutarel O. Acquired heart conditions in adults with congenital heart disease: a growing problem. Heart 2014;100:1317-21. [Crossref] [PubMed]