
Antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention, including compliance with current guidelines—data from the POLish Atrial Fibrillation (POL-AF) Registry
Introduction
Most patients with atrial fibrillation (AF) require chronic oral anticoagulation (OAC) to prevent thromboembolic events, including stroke. Moreover, almost 20–30% of them suffer from coronary artery disease (1-3). In consequence, the AF population accounts for approximately 5% to 7% of patients undergoing percutaneous coronary intervention (PCI) (4). For many years, the combination of dual antiplatelet therapy (DAPT) and OAC (triple antithrombotic therapy, TAT) was recommended by the European Society of Cardiology (ESC) in patients with AF undergoing PCI. Recent randomized clinical trials (PIONEER AF-PCI, WOEST, RE-DUAL PCI, ENTRUST AF-PCI, and AUGUSTUS) demonstrated the benefits of OAC and single antiplatelet therapy (dual antithrombotic therapy, DAT), which, with comparable antithrombotic efficacy, reduced the frequency of major bleeding in patients undergoing PCI (5-9). The most recent guidelines step forward by shortening the time of TAT, even if recommended (10,11).
Another important issue is the selection of appropriate OAC doses. In particular, in accordance with the guidelines that recommend the use of non-vitamin K antagonist oral anticoagulant (NOAC) instead of vitamin-K antagonists (VKAs), the use of NOACs in the treatment of patients with AF has grown rapidly in recent years (10,11). However, the reduction of NOAC doses is recommended in some clinical settings. A recent large meta-analysis showed that in eligible patients, such a treatment strategy, compared to the use of warfarin, was associated with a decreased risk of bleeding and complications such as stroke or systemic embolism (12).
This study assesses the everyday practice of 10 cardiology departments in antithrombotic therapy in a nationwide cohort of hospitalized patients with AF undergoing elective or urgent PCI and its agreement with current guidelines. Additionally, we estimate how often reduced NOAC doses were used during combination antithrombotic therapy and whether a low NOAC dose was applied according to ESC guidelines.
We present the following article in accordance with the STROBE reporting checklist (available at: http://dx.doi.org/10.21037/cdt-20-839).
Methods
The study was conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of the Swietokrzyska Medical Chamber in Kielce (104/2018). The Ethics Committee waived the requirement of obtaining informed consent from the patients to participate in the study.
Study design and patients
Our study was a part of a prospective, observational, multicenter registry based on the medical data of consecutive AF patients who were admitted to Polish cardiology departments between January and December 2019 (The POLish Atrial Fibrillation, POL-AF). Patients were included in the register within two selected weeks of every month (full weeks, seven days), excluding patients scheduled for ablation (usually these patients are younger and do not have concomitant diseases). Patients with AF diagnosed on admission to hospital or during hospitalization, including patients with potentially reversible causes of arrhythmia, were included in the registry. No explicit exclusion criteria were defined to avoid a biased selection of patients and achieve a cohort close to “real life”. From the whole group of patients with AF mentioned above, we separated those who underwent PCI during hospitalization [elective PCI or performing due to acute coronary syndrome (ACS)].
The collected data included baseline demographic characteristics, the results of a clinical evaluation, laboratory tests, echocardiography, and the treatment strategy recommended upon discharge with a particular emphasis on an antithrombotic treatment strategy. The clinical evaluation focused on age, sex, co-morbidities, type of AF, type and doses of anticoagulants. CHA2DS2VASc and HASBLED scores were calculated for each patient according to the recommendations (13,14). Laboratory tests included the evaluation of renal function [estimated glomerular filtration rate (eGFR), creatinine] and morphology parameters. eGFR was calculated from the Modification of Diet in Renal Disease or Chronic Kidney Disease Epidemiology Collaboration formula. The analyzed echocardiographic parameter was the left ventricular ejection fraction (LVEF).
Type of antithrombotic therapy in AF patients undergoing PCI
An analysis of the antithrombotic therapy was performed in the whole group and separately in a group of AF patients undergoing elective PCI—“the elective-PCI group” and urgent PCI during ACS—“the ACS group.”
Recommended antithrombotic therapy was defined as therapy rigorous according to recommendations, which means TAT (DAPT + OAC) or DAT, defined as OAC + single antiplatelet agent, if the patient had a high risk of bleeding complications indicated by HASBLED ≥3 points (13-16).
Other types of treatment were divided into two groups. The first group called “Lenient antithrombotic therapy” was defined as treatment using DAT despite indications for TAT, using only DAPT, using single antithrombotic therapy, and no antithrombotic therapy. The second group called “Aggressive antithrombotic therapy” included those using TAT despite permissible DAT treatment.
Use of NOACs as a part of combined therapy in AF patients undergoing PCI
An analysis of the application of the OAC type of patients was conducted. The use of reduced NOAC doses during combined treatment in post-PCI patients with AF was specifically evaluated, analyzing the compliance of dose reduction with the ESC guidelines (10,11,15,16) and the Summary of Product Characteristics registered in European Medicine Agency (EMA) (17-19). According these mentioned above documents patients were categorized by the usage of a reduced NOAC dose: appropriate reduced dose, inappropriate low dose, and inappropriate high dose. Approved dose criteria were specific for each NOAC according to the following patient characteristics: weight, age, renal function, and concomitant medications.
Statistical analysis
The statistical analysis was performed using Statistica 12.0 (StatSoft, Inc., Tulsa, OK, USA). The distribution and normality of the data were assessed by visual inspection and the Kolmogorov-Smirnov test. Continuous variables were presented as means ± standard deviations (SD) and categorical variables as absolute and relative frequencies (percentages). To analyze the differences between subgroups, the Student’s t-test for normally distributed data and the Mann-Whitney U-test if the data were not normally distributed were applied. For categorical variables, the chi square test and Fisher exact test were used. A P value of <0.05 indicated statistical significance.
Results
Of the 3,999 POL-AF registry patients, 364 (9.1%) underwent PCI during hospitalization. Table 1 presents other reasons for the hospitalization of patients with AF. Five patients died before discharge from hospital (4 with ACS). The remaining 359 patients were subjected to the final analysis: 148 (41%) patients with urgent PCI due to ACS (the ACS group) and 211 (59%) patients with elective PCI (the elective-PCI group).

Full table
The average age of the entire study group was 73.3 years [235 (65.7%) of men]. The main comorbidities in the study group were heart failure (246; 68.5%), hypertension (318; 88.6%), diabetes mellitus (164; 45.7%), history of myocardial infarction (182; 50.7%), previous PCI (196; 54.6%), and chronic kidney disease (102; 28.4%). Table 2 presents the detailed characteristics of the study group.
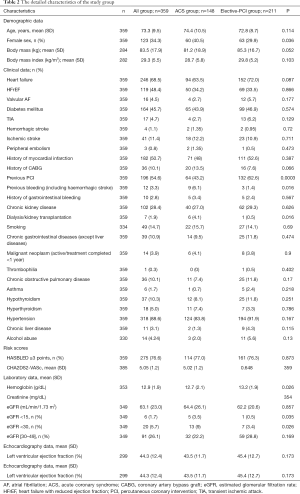
Full table
The group of patients undergoing PCI due to ACS compared to those with elective-PCI presented more often a history of bleeding, dialysis, or kidney transplantation, decreased eGFR (<30 mL/min/1.73 m2), and lower hemoglobin level. The proportion of females was higher and the previous history of PCI was lower in this group.
Analysis of antithrombotic treatment strategy in ACS-group vs. elective-PCI group
TAT was used more frequently in both patient groups than recommended by the guidelines. An analysis of TAT showed that 80 patients in the ACS group and 120 patients in the elective-PCI group were treated with TAT, despite guidelines allowing for treatment reduction to DAT (i.e., aggressive antithrombotic therapy). Lenient antithrombotic therapy was found much less frequently in 31 of the ACS patients and 63 of the elective-PCI patients. Figures 1 and 2 show detailed antithrombotic therapy data for both groups. Figure 3 presents detailed OAC therapy in the study group.
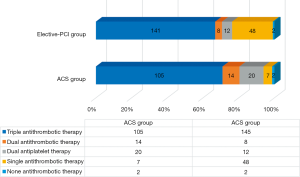
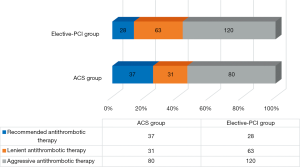
Antithrombotic therapy with OAC and ticagrelor or prasugrel was not reported in our patients. Ticagrelor plus ASA was used in three patients (one in the elective-PCI group and two in the ACS group).
Analysis of the use of NOAC in AF patients undergoing PCI
In the study group, 41 patients (11.4%) were treated with VKAs, 11 were diagnosed with valvular AF, and four were treated with NOACs despite the diagnosis of valvular AF.
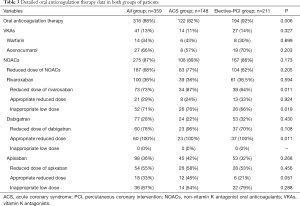
Table 3 and Figure 4 present detailed results of the analysis regarding the use of NOACs (including reduced doses of them) in the entire group of patients and separately for the two groups.
Full table
The analysis shows that patients received reduced doses of NOACs much more frequently than recommended, especially apixaban and rivaroxaban. In total, reduced doses of NOAC were used in 73 (73%) of patients for rivaroxaban and in 54 (55%) of patients for apixaban, but the percentage of inappropriate low doses of NOACs was 52 (71%) for rivaroxaban and 36 (67%) for apixaban. The problem of using inappropriate high doses of NOAC was minor and related mainly to dabigatran. In the case of dabigatran, all patients received a reduced dose of NOACs in agreement with the guidelines. However, 21% were recommended a full dose of dabigatran despite indications to reduce it.
The subjects treated with lower doses of rivaroxaban/apixaban were, as a result of EMA guidelines, older, more frequently with chronic kidney disease, and presenting a lower renal filtration rate and lower body mass index. However, the inappropriate low dose group also showed a higher prevalence of ischemic stroke and higher hemoglobin concentration (see Table 4). An exemplary age-dependency analysis proved that an inappropriately low dose of apixaban is frequently used not only in patients ≥80 years (as the only one of minimum two indications to dose reduction) but also in middle-aged (65–79 years) patients (Figure 5).
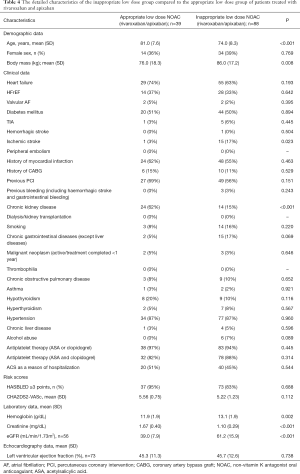
Full table
A comparison of the ACS group and the elective-PCI group showed that the use of reduced NOAC doses was comparable in both groups (83–77% vs. 104–62%; P=0.2; see Table 3).
Discussion
Decisions on how to treat patients with AF who are undergoing PCI remain challenging. Many patients with AF require NOAC treatment. Moreover, as our recent study showed, the prevalence of left atrial appendage thrombus in AF patients with lower class (IIa) recommendation to anticoagulants was comparable to the higher one (I class) (20). Doctors have to choose between bleeding risk on the one hand and thromboembolic risk on the other. The findings of our real-life registry showed that some AF patients discharged from the hospital after a PCI procedure receive TAT despite an increased risk of bleeding. The study by Bogacki et al. (21) indicated that TAT is associated with high bleeding rates and mortality in those patients. These complications were observed after a relatively short period. Similarly, other authors in a meta-analysis of six studies and comparing the use of TAT vs. DAT confirmed that reducing treatment from TAT to DAT leads to a reduced bleeding risk without affecting the incidence of thromboembolic events (22). In our registry, 80 patients in the ACS-group and 120 patients in the elective-PCI group were treated too aggressively with TAT despite guidelines allowing their treatment with DAT. Recent evidence from randomized clinical trials showed (5-9) that DAT seems to be the optimal balance between protecting patients against thromboembolic events and avoiding unnecessary bleeding complications.
In our registry, we do not record data on possible bleeding complications in patients treated too aggressively. Therefore, we were not able to verify previous studies (6,7,21) reporting that the use of TAT in AF patients significantly increases the risk of bleeding complications. Additionally, we did not investigate all potentially co-existing high-risk features for ischemic events mentioned in the guidelines (11), such as diffuse multivessel disease, unfavorable coronary anatomy, and complex revascularization. In some cases, it could explain the aggressive approach to antithrombotic treatment. However, the observed high percentage of aggressively treated patients suggests a significant influence of local practices that should be carefully verified.
Predictably, significantly noticeable in our registry was a trend of using NOACs instead of VKAs. In the present study, nearly 90% of patients treated with OAC received NOACs. This trend is common in many countries where the use of NOACs is increasing at the expense of VKAs. A recent large meta-analysis (23) aimed to assess benefits and risks associated with the use of NOACs versus VKAs in AF patients undergoing PCI, with a particular focus on the combination of antithrombotic therapy. The authors unequivocally showed that combined antithrombotic therapy with NOACs (both as DAT or TAT) is safer than with VKAs with respect to bleeding risk and results in no increase in thromboembolic events. Moreover, the magnitude of the effect was larger when NOACs were used in DAT than TAT. These findings were confirmed in the other meta-analysis, which included more than 10,000 patients (participants of four randomized trials: WOEST, PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS) (24). The use of NOACs was associated with less bleeding compared to VKAs plus DAPT. Moreover, the authors suggested that using VKAs plus DAPT should generally be avoided. Further, Chi et al. (25) reported that all combination therapies with NOACs were superior in terms of bleeding and noninferior efficacy compared to VKAs.
The studies mentioned above show that the advantage of using NOAC in combination antithrombotic therapy is mainly due to a lower frequency of bleeding complications.
Another interesting point that could potentially influence the prognosis, is the dose adjustment of NOAC. All types of NOAC require dose adjustments based on certain clinical criteria. Notably, in most of the published RCTs, the dose of NOACs was reduced as recommended by FDA/EMA. In the PIONEER-AF trial, rivaroxaban was reduced from 15 to 10 mg for patients with decreased creatinine clearance. Additionally, in the ENTRUST trial, edoxaban was reduced if moderate or severe renal impairment was observed. Finally, in the AUGUSTUS trial, the dose of apixaban was reduced according to the recommendations (depending on age, weight, and creatinine level). The treatment of such patients is challenging in terms of obtaining benefits while reducing complications. In our registry, we observed examples of both over- and undertreatment. In the case of dabigatran, a full dose was used in 21% of people with indications for its reduction. However, no patient was treated with reduced dabigatran without substantiation. On the other hand, underdosing was noticeable for apixaban and rivaroxaban (in 71% and 67% patients, respectively). Underdosing apixaban/rivaroxaban in our study is evidently more frequent than in other reports. In the study by Steinberg et al. (26), only 9.4% of patients were underdosed, 3.4% were overdosed, and all had an increased risk of cardiovascular events. In a single-study registry by Sato et al. (27), 23% of AF patients were treated with inappropriate low doses of NOAC, and clinical factors such as age and creatinine clearance identified patients at risk of underdosed NOAC. In our study, the factors predisposing to using an inappropriate low dose of NOAC were mostly comparable to those shown in the studies mentioned above. In the case of age, kidney function, and body mass index, the most probable explanation is that reduced doses of rivaroxaban and apixaban are used even if the cut-offs for these parameters, which are mentioned as indication for a lower dose, are not reached. An exemplary analysis showing the dependence of an inappropriately low dose of apixaban on age depicts this phenomenon—a reduced dose was most frequently used in the elderly [even with a mass over 60 kg and creatinine below 1.5 mg/dL (133 µmol/L)] than in slightly younger (65–79 years) subjects. Other authors also revealed the problem with using anticoagulation therapy in the patients with chronic kidney disease (28). In their study, OAC was less frequent used in the patients with kidney disease (defined as eGFR ≤60 mL/min/1.73 m2), even although the higher CHA2DS2VASc score. Moreover, in our analysis, an inappropriate low dose of NOAC was much more frequently used in patients after an ischemic stroke. We can only hypothesize that some clinical features, such as neurological defects and a higher risk of falling, may prompt the reduction of OAC in those subjects. The explanation for higher hemoglobin concentration as a factor predisposing to inappropriately low doses of NOAC is unclear and is probably a secondary statistical effect related to other factors discriminating both groups, with no clinical meaning.
Strengths and limitations
Our prospective multicenter registry shows real-life therapeutic decisions made in AF patients undergoing PCI in many Polish departments. It assesses their compliance with the guidelines and thus reveals the practices that should be carefully verified; however, it also has several limitations. First, we do not have detailed information about the periprocedural characteristics with respect to the coronary anatomy and PCI techniques, which limits the possibility of assessing the compliance of used antithrombotic therapy with the current guidelines. Second, due to the lack of long-term follow-up, it was not possible to assess long-term complications that may result from the applied treatment. Finally, we realized that the group of our patients is quite small and the larger study is needed to check the results obtained in our “pilot study”.
Conclusions
In patients with AF undergoing PCI, NOACs are definitely preferred over VKAs in TAT/DAT and an aggressive antithrombotic strategy with TAT is frequently chosen even if DAT is permissible by the guidelines. Label adherence of a reduced NOAC dose during combination therapy is not satisfactory for apixaban and rivaroxaban and probably results from too cautious an approach to the known indications for reduced therapy.
Acknowledgments
The POL-AF registry was initiated on the Scientific Platform of the “Club 30” of the Polish Cardiac Society. Investigators other than those listed as Authors include: Monika Budnik (Warsaw), Katarzyna Karoń (Warsaw), Monika Szewczak (Warsaw), Bartosz Krzemiński (Grodzisk Mazowiecki), Wiktor Wójcik (Warsaw).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at: http://dx.doi.org/10.21037/cdt-20-839
Data Sharing Statement: Available at: http://dx.doi.org/10.21037/cdt-20-839
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-839). IG reports personal fees from Bayer and Boehringer-Ingelheim; AKC reports honoraria for lectures from Bayer, outside the submitted work; ATK reports research grant from Boehringer-Ingelheim, consultant for Boehringer-Ingelheim, Bayer, speaker for Boehringer-Ingelheim; BWK reports personal fees from Boehringer-Ingelheim, Bayer, Pfizer, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of the Swietokrzyska Medical Chamber in Kielce (104/2018). The Ethics Committee waived the requirement of obtaining informed consent from the patients to participate in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kralev S, Schneider K, Lang S, et al. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS One 2011;6:e24964. [Crossref] [PubMed]
- Nieuwlaat R, Capucci A, Camm AJ, et al. Atrial fibrillation management: A prospective survey in ESC member countries: The euro heart survey on atrial fibrillation. Eur Heart J 2005;26:2422-34. [Crossref] [PubMed]
- Nabauer M, Gerth A, Limbourg T, et al. The registry of the German competence NETwork on atrial fibrillation: Patient characteristics and initial management. Europace 2009;11:423-34. [Crossref] [PubMed]
- Angiolillo DJ, Goodman SG, Bhatt DL, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention. A North American perspective-2016 update. Circ Cardiovasc Interv 2016;9:e004395. [Crossref] [PubMed]
- Gibson CM, Mehran R, Bode Ch, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375:2423-34. [Crossref] [PubMed]
- Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013;381:1107-15. [Crossref] [PubMed]
- Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513-24. [Crossref] [PubMed]
- Vranckx P, Lewalter T, Valgimigli M, et al. Evaluation of the safety and efficacy of an edoxaban-based antithrombotic regimen in patients with atrial fibrillation following successful percutaneous coronary intervention (PCI) with stent placement: Rationale and design of the ENTRUST-AF PCI trial. Am Heart J 2018;196:105-12. [Crossref] [PubMed]
- Lopes RD, Vora AN, Liaw D, et al. An Open-label, 2 x 2 factorial, randomized controlled trial to evaluate the safety of apixaban vs. vitamin K antagonist and aspirin vs. placebo in patients with atrial fibrillation and acute coronary syndrome and/or percutaneous coronary intervention: Rationale and design of the AUGUSTUS trial. Am Heart J 2018;200:17-23. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease Developed in Collaboration with EACTS: The Task Force for Dual Antiplatelet Therapy in Coronary Artery Disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213-60. [Crossref] [PubMed]
- Wang X, Fang L, Liu B, et al. Real-world comparisons of reduced-dose non-vitamin K antagonist oral anticoagulants versus warfarin in atrial fibrillation: a systematic review and meta-analysis. Heart Fail Rev 2020;25:973-83. [Crossref] [PubMed]
- Lip GYH, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro heart survey on atrial fibrillation. Chest 2010;137:263-72. [Crossref] [PubMed]
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro heart survey. Chest 2010;138:1093-100. [Crossref] [PubMed]
- Knuuti J, Ijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. The task force for the diagnosis and management of chronic coronary syndromes of the European society of cardiology (ESC). Eur Heart J 2020;41:407-77. [Crossref] [PubMed]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020;ehaa612.
- “Pradaxa,” European Medicines Agency, last updated June 6, 2020. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/pradaxa
- “Xarelto,” European Medicines Agency, last updated January 17, 2020. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xarelto
- “Eliquis,” European Medicines Agency, last updated May 19, 2020. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/eliquis
- Uziębło-Życzkowska B, Krzesiński P, Jurek A, et al. Prevalence and risk factors of left atrial thrombus in patients with atrial fibrillation and lower class (IIa) recommendation to anticoagulants. Cardiovasc Diagn Ther 2020;10:717-24. [Crossref] [PubMed]
- Bogacki P, Kabłak-Ziembicka A, Bryniarski K, et al. Triple anticoagulation therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention - Real life assessment. Postepy Kardiol Interwencyjnej 2016;12:303-13. [Crossref] [PubMed]
- D’Ascenzo F, Taha S, Moretti C, et al. Meta-analysis of randomized controlled trials and adjusted observational results of use of clopidogrel, aspirin, and oral anticoagulants in patients undergoing percutaneous coronary intervention. Am J Cardiol 2015;115:1185-93. [Crossref] [PubMed]
- Eyileten C, Postula M, Jakubik D, et al. Non-vitamin K oral anticoagulants (NOAC) versus vitamin k antagonists (VKA) for atrial fibrillation with elective or urgent percutaneous coronary intervention: A meta-analysis with a particular focus on combination type. J Clin Med 2020;9:1120. [Crossref] [PubMed]
- Lopes RD, Hong H, Harskamp RE, et al. Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention: A network meta-analysis of randomized controlled trials. JAMA Cardiol 2019;4:747-55. [Crossref] [PubMed]
- Chi G, Kerneis M, Kalayci A, et al. Safety and efficacy of non-vitamin K oral anticoagulant for atrial fibrillation patients after percutaneous coronary intervention: A bivariate analysis of the PIONEER AF-PCI and RE-DUAL PCI trial. Am Heart J 2018;203:17-24. [Crossref] [PubMed]
- Steinberg BA, Shrader P, Thomas L, et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: The ORBIT-AF II registry. J Am Coll Cardiol 2016;68:2597-604. [Crossref] [PubMed]
- Sato T, Aizawa Y, Fuse K, et al. The comparison of inappropriate-low-doses use among 4 direct oral anticoagulants in patients with atrial fibrillation: From the database of a single-center registry. J Stroke Cerebrovasc Dis 2018;27:3280-8. [Crossref] [PubMed]
- Tomaszuk-Kazberuk A, Nikas D, Lopatowska P, et al. Patients with Atrial Fibrillation and Chronic Kidney Disease More Often Undergo Angioplasty of Left Main Coronary Artery - a 867 Patient Study. Kidney Blood Press Res 2018;43:1796-805. [Crossref] [PubMed]





