A new polymer-free drug-eluting stent with nanocarriers eluting sirolimus from stent-plus-balloon compared with bare-metal stent and with biolimus A9 eluting stent in porcine coronary arteries
Introduction
Permanent polymers of first generation drug-eluting stents (DES) are imputed to be a contributing factor in vessel wall inflammation, positive remodeling, stent malapposition and ultimately late stent thrombosis (1-3). New DES with biodegradable polymers and DES with no polymers have been developed and tested in preclinical and clinical experiments to overcome the limitations of first generation DES with durable polymers.
Polymeric coatings was thought to be essential for effective local drug delivery to the vessel wall, since its absence has been related to target vessel failure (4). However, many recent studies with surface-modified polymer-free DES showed that the elimination of polymers might not compromise the anti-restenosis efficacy of DES (5,6). Accordingly, new polymer-free DES has been deve loped by a number of stent manufactures by texturizing, creating grooves, niches, or a microporous stent surface (7,8).
This article describes the experimental results of the novel Focus np stent (Envision Scientific Pvt. Ltd., Gujarat, India), a polymer-free stent developed with nanotechnology that uses nanosized phospholipid particles to deliver sirolimus from a combined balloon-plus-stent platform.
Methods
Animal study
Animal experiments were in compliance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences and National Research Council. National Academy Press, Washington, D.C., 1996), and the study protocol was approved by the Institutional Ethics Committee. Stents were implanted in 10 juvenile domestic pigs, weighing 23 to 30 kg, and animals were followed for 28 days. Oral aspirin (100 mg) and clopidogrel (300 mg loading dose; 75 mg daily dose) were administered starting 1 day before the procedure and continued daily until euthanasia. Animals were sedated and anesthetized with an intramuscular solution of midazolam (0.5 mg/kg) and ketamine (3 mg/kg) followed by intravenous thiopental (25 mg/kg). Mechanical ventilation was established and anesthesia maintained with inhaled isoflurane (2% to 5%) throughout the procedure. Arterial access was achieved by a femoral cut-down with a general sterile technique, and coronary angiography was then performed using 6F guiding catheters after intracoronary administration of nitroglycerin (200 µg) and intravenous heparin (7,500 IU). Three types of stents were implanted: (I) cobalt-chromium polymer-free sirolimus eluting Focus np stent; (II) stainless steel biolimus A9-eluting Biomatrix stent (Biosensors International, Singapore) and (III) a cobalt-chromium bare metal stent (BMS) Amazonia CroCo® stent, (Minvasys, Paris, France). All stents were 3.0 mm in diameter, and stent length was 16 mm for polymer-free sirolimus eluting Focus np stent, 14 mm for biolimus A9 eluting Biomatrix stent, and 16 mm for Amazonia CroCo® BMS. For each animal, the study stents were deployed according to a randomization chart (1:1:1) in the major branches of the coronary arteries (left anterior descending coronary artery, left circumflex artery, and right coronary artery) in a non-tapered, non-bifurcation, non-angulated segment of 3.0 mm in diameter by visual estimation. The stents were implanted through inflation of the delivery balloons for approximately 30 seconds in all study groups.
The guiding catheter was used as a reference to obtain a 1.1:1 to 1.2:1 stent-to-artery ratio compared with the baseline vessel diameter and to induce moderate vessel injury (9). Animals were allowed to recover and received appropriate postoperative care. At 28-day follow-up, control coronary angiography and optical coherence tomography (OCT) were performed, and the animals under deep anesthesia were euthanized with a lethal dose of potassium chloride.
Design of Focus np stent
The Focus np stent is a cobalt-chromium L-605 stent with strut thickness of 73 µm. It is crimped onto a COPAN Co-Polyamide delivery balloon. Nanoparticles with a mean diameter of 210 nm, containing sirolimus (108 µg of sirolimus on a 3.0×16 mm stent), were synthesized using an ultrasonic homogenizer and applied using an inert gas-assisted spray process in stents already crimped onto the balloon delivery system. Consequently, the coating was uniformly distributed over the stent-plus-balloon surface, with only the abluminal face of the stent struts having the coating applied (Figure 1). Focus np stent thus, is a polymer-free cobalt-chromium stent delivering sirolimus through nanoparticles from balloon and abluminal stent strut face. The manufacturing and coating process of sirolimus nanoparticles to metallic stent platform and the balloon-catheter delivery system is described in detail elsewhere (10). In a previous experiment in iliac arteries of rabbits treated with a 60 s dilatation with sirolimus-coated balloons, these nanoparticles were found initially in the endothelial surface, then in the medial layer and finally in the deeper stratum like the adventitia (11). In vitro drug release analyzed using HPLC revealed that sirolimus is delivered from the stent with an initial burst followed by a prolonged release for up to 40 days. In-tissue release of sirolimus from phospholipid nanoparticles is longer because of encapsulation in drug delivery matrix (10).
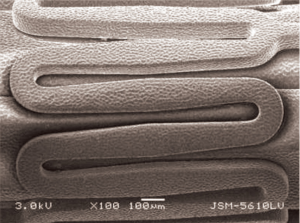
Description of control stents
Amazonia CroCo (Minvasys, France) is a cobalt-chromium BMS with a thin strut thickness of 73 µm and a design that combines intermediate and open cells. This stent is used as the metallic platform in the Amazonia Pax paclitaxel polymer-free DES (12).
Biomatrix (Biosensors International, Singapore) is a DES that consists of a stainless steel platform with a strut thickness of 112 µm and a quadrature link design. It is coated exclusively in the abluminal stent surface with a biodegradable polymer (PLA, polylactic acid) and biolimus A9 (218 µg on a 3.0×14 mm stent). This biodegradable coating completely dissolves into carbon dioxide and water after a 6- to 9-month period. Biolimus A9 is a semi-synthetic sirolimus analogue with 10 times higher lipophilicity and similar potency as sirolimus (13,14).
Optical coherence tomography (OCT)
At 28-day follow-up and after control angiography, a 0.014-inch guidewire was inserted into the coronary arteries, and an occlusion balloon (Helios™, LightLab Imaging) was advanced distally to the treatment sites. The 0.014-inch wire was then removed and replaced for a 0.016-inch OCT catheter (Image Wire™, LightLab Imaging, Westford, MA, USA). The Helios™ balloon (LightLab® Imaging) was pulled back proximally to the stent and inflated with a 1:1 saline:contrast solution until occlusion of the artery. Then, saline was flushed to clear the lumen from blood and an automatic pullback of the OCT catheter was initiated at a speed of 1 mm/s with recording of OCT images. These images were analyzed for thrombus, strut malapposition and endothelialization.
A strut was considered malapposed if the distance from the endoluminal surface of the stent to the vessel wall was higher than the sum of the metal and, if present, the polymer thickness. The cutoff points used for each stent type were: BMS (Amazonia CroCo) 73 µm, polymer-free sirolimus-eluting stent (Focus np) 73 µm, biolimus-eluting stent (Biomatrix) 120 µm. A stent strut was defined as covered, if there was a visible layer of tissue covering it.
Semi-automatic measurements were made with proprietary software for off-line analysis with the M2 System (LightLab Imaging, Westford, MA, USA) to determine lumen area, stent area, neointimal thickness (distance from stent strut until lumen), neointimal area (stent area minus lumen area) and percentage of neointimal area (ratio of neointimal area and stent area, multiplied by 100) (15).
Light microscopy
Immediately after euthanasia, the hearts were excised and the coronaries were pressure-perfused (~100 mm Hg) via the ascending aorta with 0.9% saline, to clear the blood, followed by perfusion of 10% buffered formalin for a minimum of 1 hour. The stented arterial segments were then excised from the heart by dissection and fixed by immersion in 10% formalin overnight. The artery-stent specimens were dehydrated with ethanol solutions of increasing concentrations and embedded in methyl methacrylate resin. A total of three cross-sections (proximal, mid, and distal) were obtained from each vessel on a rotary microtome (cut thickness 3.5 µm) and stained with hematoxylin and eosin and Verhoeff stains for elastic fibers.
Microscopic digital morphometry with Leica QWin® software (Leica Microsystems, Wetzlan, Germany) was used to measure areas of the external elastic lamina (EEL), internal elastic lamina (IEL), stent, and lumen. Media area was defined as EEL area—EL area. Neointimal hyperplasia area was defined as IEL area—lumen area. Percent neointimal burden was defined as 100× (neointimal area/IEL area). Neointimal thickness was measured at the stent strut and at the inter-strut space.
The cross-sections were examined for semi-quantitative analysis of endothelialization, inflammation, fibrin content, angiogenesis, and vessel injury according to previously described scoring schemes (16-19). Stent endothelialization score was defined as the extent of the circumference of the arterial lumen covered by endothelial cells and graded from 1 to 3 (1=25%, 2=25% to 75%, 3≥75%). Inflammation was graded as 0, none; 1, scattered inflammatory cells; 2, inflammatory cells encompassing 50% of a strut in at least 25% to 50% of the circumference of the artery; 3, inflammatory cells surrounding a strut in at least 25% to 50% of the circumference of the artery. Inflammation modified was graded as 0, no inflammatory cells surrounding the strut; 1, light, non-circumferential lymphohistocytic infiltrate surrounding the strut; 2, localized, moderate to dense cellular aggregate surrounding the strut non-circumferentially; 3, circumferential dense lymphohistiocytic cell infiltration of the strut. The intimal fibrin content was graded as 0, no fibrin deposition; 1, focal residual fibrin involving any portion of the artery or moderate fibrin deposition adjacent to the strut involving <25% of the circumference of the artery; 2, moderate fibrin involving >25% of the circumference of the artery or heavy deposition involving <25% the circumference of the artery; or 3, heavy deposition of fibrin involving >25% of the circumference of the artery. The injury score (Schwartz) was graded as 0, internal elastic lamina intact, media compressed but not lacerated; 1, internal elastic lamina lacerated, media typically compressed but not lacerated; 2, internal elastic lacerated, media visibly lacerated, external elastic lamina intact but compressed or 3, external elastic lamina lacerated, typically large lacerations of media extending through the external elastic lamina, stent struts sometimes residing in adventitia. The injury score (Gunn) was graded as 0, no impression of metal upon media; 1, deformation of the internal elastic lamina by <45°; 2, deformation of the internal elastic lamina by >45°; 3, rupture of the internal elastic lamina or 4, rupture of the external elastic lamina (i.e., complete medial rupture).
Statistical analysis
Data are presented as mean ± SD and/or median (interquartile range; IQR). The analyzed per stent individual OCT frames and histologic cross-sections were treated as independent samples (7). Differences in continuous variables among stent groups were evaluated by using parametric (analysis of variance) or non-parametric (Kruskal-Wallis) statistical tests as needed. Histologic ordinal variable results were overall compared for differences among stent groups with independent-samples Kruskal-Wallis test. The statistical software SPSS 20.0 was used to analyze data, and a priori P value ≤0.05 was considered significant.
Results
Twenty-three stents (8 Amazonia CroCo, 8 Focus np, and 7 Biomatrix) were successfully implanted in the coronary arteries of 10 swine. Mean balloon pressure to implant the stents was equal among groups (P=0.895). Nine of 10 animals survived until the follow-up phase of the study. There was one early death (pig number 2) that occurred during the second day after the stent implantation procedure (Biomatrix stent implanted in the left anterior descending artery and Amazonia CroCo in the left circumflex artery). In this animal, a stent was implanted too distally in the coronary tree, resulting in dissection at the distal stent edge that was managed by prolonged balloon inflation with final TIMI III flow. Pathology revealed the presence of a recent intraluminal thrombus in all stents. The remaining 9 pigs with 21 stents (7 Amazonia CroCo, 8 Focus np, and 6 Biomatrix) were included in the study for final analysis. Stent distribution is summarized in Table 1. At 28-day follow-up, all stents in surviving animals were patent at angiography and could be imaged with OCT.
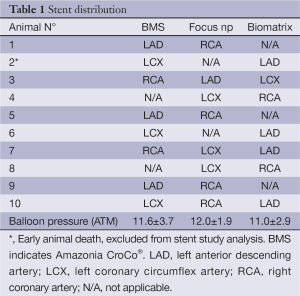
Full table
OCT findings
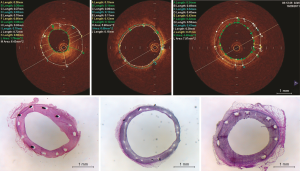
OCT quantitative analysis is summarized in Table 2, and illustrative example OCT images of each group are shown in Figure 2 (upper panel). At 28 days, lumen area was larger in Focus np compared with BMS and Biomatrix. Neointimal hyperplasia thickness was reduced in Focus np compared with BMS and Biomatrix. NIH area and percentage NIH were smaller in Focus np than BMS and Biomatrix. Biomatrix stent showed either a less-pronounced neointimal thickness or hyperplasia area than BMS. Stent struts were completely covered and well apposed in all stent groups.
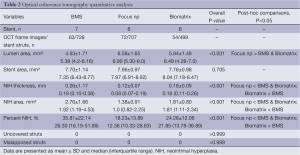
Full table
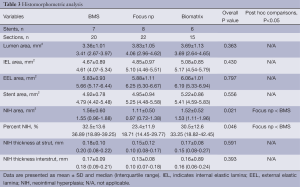
Histomorphometric and semiquantitative histologic analysis
The histomorphometric analysis is summarized in Table 3, and illustrative example histologic images of each group are shown in Figure 2 (bottom panel). Lumen area, internal elastic lamina area, external elastic lamina area, stent area, and neointimal thickness at strut and interstrut were not different among groups. The Focus np stent had a smaller neointimal area and percentage neointimal area compared with BMS only.
Full table
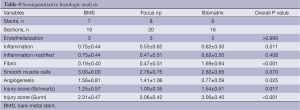
Semiquantitative histologic analysis revealed that Focus np stents were associated with an intermediate degree of fibrin content, smaller than the Biomatrix but higher than the control BMS (Table 4). In addition, Focus np stents also had an intermediate degree of angiogenesis, smaller than the BMS but higher than the Biomatrix stent. Even though the delivery pressure and the tentative balloon:artery ratio (1.1:1.0) was the same for all implants, the vessel injury score was higher in Biomatrix stent group.
Full table
Discussion
In this preclinical experiment, the polymer-free Focus np balloon-plus-stent coated with sirolimus-containing nanoparticles had an excellent safety profile, yielding good results at 28 days after implantation in pig coronaries evaluated by OCT and histology. Due to a unique patented manufacture process that allows Focus np to elute sirolimus from a stent-plus-balloon platform, therefore delivering drug to the entire vessel circumference, there is a possibility that this device might be more effective in inhibiting neointimal growth than existing commercial DES eluting antiproliferative drugs from stent surface alone. Indeed, in the present study, the antiproliferative effect of Focus np assessed by OCT was significantly higher than the clinically available Biomatrix DES, which elutes drug exclusively from the abluminal stent strut surface.
Related to the comparison of the Focus np stent with the Biomatrix stent, although these two stents are markedly different regarding metallic platform, presence or absence of polymer, and differing antiproliferative agents, the Biomatrix stent data were useful as a “positive” control group, enabling us a direct comparison with this successful clinically used DES.
A relevant finding in our study was the higher fibrin content in the histologic sections of the Biomatrix stent compared to the Focus np stent and to BMS. This suggests a more delayed healing process in the biolimus A9 eluting stent. Of note, in a preclinical experiment comparing the high-dose biolimus A9 stent (225 µg/14 mm), low-dose biolimus A9 (112 µg/14 mm) polymer-free Biofreedom stent (Biosensors International, Singapore), polymer-coated sirolimus-eluting Cypher stent, and BMS in porcine coronary arteries, histological evaluation showed a higher fibrin deposition for all these 3 DES groups than BMS at 28 days (7).
Sirolimus is a lipophilic drug with potent antiproliferative and immunosuppressant effects. There are some technical difficulties in coating a balloon or a balloon-plus-stent and effectively delivering this drug into the arterial wall tissue without a durable or a biodegradable polymer. In a sirolimus eluting polymer-free DES, a fraction of the sirolimus load can be removed from the device when passing through the hemostatic valve, delivery catheter, calcified lesions, or be simply washed out by the blood stream. Encapsulation of sirolimus in nanoparticles to deliver it to the vessel wall through stent-plus-balloon is an attractive technology as the plaque area treated involves both balloon and stent area in contact with the vessel inner wall (stent edges included).
Many polymer-free stents have been investigated in clinical studies, including BioFreedom (Biosensors International, Singapore) (20), Cre8 (CID, Italy) (21), Yukon Choice PF (Translumina, Germany) (22), Nile Pax (Minvasys, France) (23) and VESTAsync stent (MIV Therapeutics, USA) (24). The strategies for drug binding and release without the use of polymers consists of loading antiproliferative agents (biolimus A9, amphilimus, paclitaxel, sirolimus or a combination of sirolimus and probucol) on microporous or reservoirs on stent surface.
The results of this preclinical study with Focus np stent warrants initiation of clinical studies as a First-in-Man (FIM) study and randomized clinical trials comparing it with currently approved DES.
Study limitations
The pig model of coronary stent implantation is well-known to be more suited for safety analysis than for efficacy evaluation of new devices. Therefore, the differences in neointimal proliferation seen among the groups should be interpreted with caution. The dissimilarities of the stent types and their respective delivery systems may account for some of the differences in the injury scores. It is not possible to rule out that some of the differences in the vascular response (e.g., neointimal proliferation, fibrin deposition) might have been related to a discrepancy in baseline vessel injury. An ideal experiment to assess the vessel response to this new technology—nanocarriers eluting sirolimus from stent-plus-balloon—would be testing stent types with exactly the same delivery balloons, metallic designs and stent lengths, and two DES releasing the same antiproliferative agent with the same dosage but different releasing drug mechanisms.
The occlusive method (time-domain OCT, M2 System, LightLab Imaging, Westford, MA, USA) was used in this experimental study. This method, recently replaced by the current non-occlusive method (Fourier-domain OCT, C7XR, St. Jude Medical), was extensively used in preclinical and clinical studies. Comparing these two OCT methods in an in vivo non-stented human coronary artery study, the occlusive method tended to reduce lumen dimension by 13%, with equivalent OCT image quality (25). In our study of stented porcine coronary arteries, the use of the occlusive method was well suited to analyze in-stent neointimal hyperplasia as the OCT NIH area and percentage NIH results were concordant with histomorphometry (26).
Our sample size was small, but the carefully selected arterial segments to accommodate the unique diameter of stents (3.0 mm) available for implantation, and the analysis of OCT frames and histologic sections as independent samples reduced the need of a larger sample size.
Conclusions
The Focus np stent with polymer-free sirolimus eluted from stent-plus-balloon demonstrated safety and a reduced intimal proliferation compared with BMS and Biomatrix stent in the present 28-day follow-up porcine coronary model. The new nanocarrier technology eluting sirolimus from stent-plus-balloon is promising and warrants confirmation of its safety and efficacy in future clinical studies.
Acknowledgements
We thank Richard Silva, Leonora Loppnow and Marcio Chaves for technical assistance and Ann Morcos (www.morcosmedia.com) for reviewing the manuscript. The study was supported in part by Envision Scientific PVT Ltd.
Disclosure: Celso K. Takimura, Micheli Z. Galon, Paulo S. Gutierrez and Pedro A. Lemos declare no conflict of interest. Prakash Sojitra, Ashwin Vyas and Manish Doshi works for Envision Scientific PVT Ltd.
References
- Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 2004;109:701-5. [PubMed]
- Cook S, Ladich E, Nakazawa G, et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 2009;120:391-9. [PubMed]
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007;115:1051-8. [PubMed]
- Lansky AJ, Costa RA, Mintz GS, et al. Non-polymer-based paclitaxel-coated coronary stents for the treatment of patients with de novo coronary lesions: angiographic follow-up of the DELIVER clinical trial. Circulation 2004;109:1948-54. [PubMed]
- Waksman R, Pakala R, Baffour R, et al. In vivo comparison of a polymer-free Biolimus A9-eluting stent with a biodegradable polymer-based Biolimus A9 eluting stent and a bare metal stent in balloon denuded and radiated hypercholesterolemic rabbit iliac arteries. Catheter Cardiovasc Interv 2012;80:429-36. [PubMed]
- King L, Byrne RA, Mehilli J, et al. Five-year clinical outcomes of a polymer-free sirolimus-eluting stent versus a permanent polymer paclitaxel-eluting stent: final results of the intracoronary stenting and angiographic restenosis - test equivalence between two drug-eluting stents (ISAR-TEST) trial. Catheter Cardiovasc Interv 2013;81:E23-8. [PubMed]
- Tada N, Virmani R, Grant G, et al. Polymer-free biolimus a9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting cypher stent in a porcine model. Circ Cardiovasc Interv 2010;3:174-83. [PubMed]
- Costa JR Jr, Abizaid A, Costa R, et al. Preliminary results of the hydroxyapatite nonpolymer-based sirolimus-eluting stent for the treatment of single de novo coronary lesions a first-in-human analysis of a third-generation drug-eluting stent system. JACC Cardiovasc Interv 2008;1:545-51. [PubMed]
- Schwartz RS, Edelman E, Virmani R, et al. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv 2008;1:143-53. [PubMed]
- Lemos PA, Farooq V, Takimura CK, et al. Emerging technologies: polymer-free phospholipid encapsulated sirolimus nanocarriers for the controlled release of drug from a stent-plus-balloon or a stand-alone balloon catheter. EuroIntervention 2013;9:148-56. [PubMed]
- Yasdani SN, Sherdiwala DP, Prakash S, et al. A temporal assessment of drug distribution following local balloon delivery of nanoparticle sirolimus. JACC 2011;58:B6.
- Capodanno D, Dipasqua F, Tamburino C. Novel drug-eluting stents in the treatment of de novo coronary lesions. Vasc Health Risk Manag 2011;7:103-18. [PubMed]
- Grube E, Hauptmann KE, Buellesfeld L, et al. Six-month results of a randomized study to evaluate safety and efficacy of a Biolimus A9 eluting stent with a biodegradable polymer coating. EuroIntervention 2005;1:53-7. [PubMed]
- Serruys PW, Farooq V, Kalesan B, et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovasc Interv 2013;6:777-89. [PubMed]
- Bezerra HG, Costa MA, Guagliumi G, et al. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc Interv 2009;2:1035-46. [PubMed]
- Suzuki T, Kopia G, Hayashi S, et al. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation 2001;104:1188-93. [PubMed]
- Carter AJ, Aggarwal M, Kopia GA, et al. Long-term effects of polymer-based, slow-release, sirolimus-eluting stents in a porcine coronary model. Cardiovasc Res 2004;63:617-24. [PubMed]
- Lowe HC, Schwartz RS, Mac Neill BD, et al. The porcine coronary model of in-stent restenosis: current status in the era of drug-eluting stents. Catheter Cardiovasc Interv 2003;60:515-23. [PubMed]
- Kornowski R, Hong MK, Tio FO, et al. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol 1998;31:224-30. [PubMed]
- Grube E, Mueller R, Schuler G, et al. TCT-46 Comparison of Polymer-Free BioFreedom™ Stents with Durable Polymer Taxus Liberté™ Stents: 3-Year Results from the BioFreedom First-In-Man Trial. J Am Coll Cardiol 2012;60: [PubMed]
- Carrié D, Berland J, Verheye S, et al. A multicenter randomized trial comparing amphilimus- with paclitaxel-eluting stents in de novo native coronary artery lesions. J Am Coll Cardiol 2012;59:1371-6. [PubMed]
- Byrne RA, Kufner S, Tiroch K, et al. Randomised trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis: 2-year follow-up results. Heart 2009;95:1489-94. [PubMed]
- Costa RA, Abizaid A, Abizaid AS, et al. Procedural and early clinical outcomes of patients with de novo coronary bifurcation lesions treated with the novel Nile PAX dedicated bifurcation polymer-free paclitaxel coated stents: results from the prospective, multicentre, non-randomised BIPAX clinical trial. EuroIntervention 2012;7:1301-9. [PubMed]
- Costa JR Jr, Abizaid A, Costa R, et al. 1-year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions: the VESTASYNC I trial. JACC Cardiovasc Interv 2009;2:422-7. [PubMed]
- Gonzalo N, Serruys PW, García-García HM, et al. Quantitative ex vivo and in vivo comparison of lumen dimensions measured by optical coherence tomography and intravascular ultrasound in human coronary arteries. Rev Esp Cardiol 2009;62:615-24. [PubMed]
- Murata A, Wallace-Bradley D, Tellez A, et al. Accuracy of optical coherence tomography in the evaluation of neointimal coverage after stent implantation. JACC Cardiovasc Imaging 2010;3:76-84. [PubMed]


