
Abnormal torsion and helical flow patterns of the neo-aorta in hypoplastic left heart syndrome assessed with 4D-flow MRI
Introduction
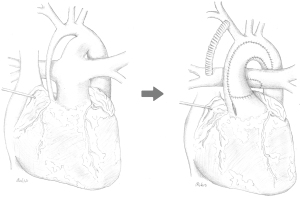
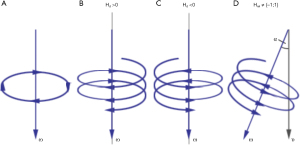
In patients with hypoplastic left heart syndrome (HLHS), formation of a “neo-aorta” is an integral part of the Norwood procedure (Figure 1) that includes creation of an anastomosis of the native aorta to the former pulmonary trunk and reconstruction of the ascending aorta and transverse arch using a porcine pericardial graft (1,2). This sophisticated aortic surgery leads to an abnormal neoaortic twist that potentially leads to abnormal fluid dynamics and increased ventricular afterload with subsequent myocardial hypertrophy and impaired ventricular function in the long run (3-5).
4D flow MRI has been shown to provide important information on fluid dynamics in the cardiovascular system (6). The agreement of 4D flow MRI with 2D phase contrast has been demonstrated previously (7). Abnormal fluid dynamics in the neoaorta of HLHS patients were quantified in a previous 4D-flow MRI study (8). Several other computational fluid dynamics (CFD) studies have also shown the impact of the aortic geometry on haemodynamics (9-11). A CFD study on aortic models with and without twist predicted a relationship between twist and helical flow patterns and motivated further studies on our patient cohort (10). No previous clinical MRI study quantified the relationship between aortic twist and helical flow patterns in HLHS patients’ neoaorta.
The present study aims to investigate the neoaorta’s abnormal twist and its consequences on fluid dynamics as well implications for the RV. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-770).
Methods
Patient data
HLHS patients were enrolled for an MRI study after 3 stage palliation (12) at the Kinderherzzentrum Kiel, Germany. The three stages of surgical palliation involved: (I) modified Norwood procedure with a Blalock-Taussig (BT)-Shunt and an aortic arch reconstruction using a porcine pericardial patch augmentation (Figure 1), (II) superior cavo-atrio-pulmonary anastomosis “Hemi-Fontan” with enlargement of the left pulmonary artery (LPA), and stage (III) Total cavo-pulmonary anastomosis by creating an intraatrial fenestrated tunnel. The patient’s ascent and parent’s consent were obtained prior to any MRI study. Healthy controls were recruited from subjects ruled out for cardiovascular disease. The subjects’ demographic characteristics are listed in Table 1. The study was approved by the institutional ethics board of Kiel University’s Faculty of Medicine (A168/07). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Table 1
| Parameter | HLHS subjects (n=42) | Healthy controls (n=11) | P value |
|---|---|---|---|
| Sex | |||
| Male | 39 (69%) | 4 (36.4%) | 0.09 |
| Female | 13 (31%) | 7 (63.6%) | |
| Age | |||
| At Norwood (days) | 5 (2.0–163) | – | – |
| At TCPC (years) | 2.55 (1.7–5.1) | – | – |
| At MRI (years) | 4.85 (2.9–17) | 12.1 (4–41.6) | 0.006 |
| Weight (kg) | 23.84±12,93 | 50.25±26.56 | <0.001 |
| Height (cm) | 116.65±22.65 | 149.02±28.84 | 0.002 |
| BSA (m2) | 0.87±0.31 | 1.42±0.52 | 0.001 |
| HR (min−1) | 84.38±10.53 | 79.82±14.32 | 0.163 |
| HLHS subtype | |||
| MA/AA | 19 (45%) | – | – |
| MS/AA | 11 (26%) | – | – |
| MA/AS | 3 (7%) | – | – |
| MS/AS | 9 (21) | – | – |
HLHS, hypoplastic left heart syndrome.
Data acquisition and reconstruction
Cardiovascular MRI examinations were performed on a 3.0 Tesla MR system (Achieva 3.0T, TX-series, Philips Healthcare, Best, The Netherlands) with a 32-channel coil for cardiac imaging. Blood flow velocities were quantified with a time-resolved phase-contrast with three-dimensional anatomic coverage (4D-flow MRI) using retrospective ECG-gating and a temporal phase resolution of 30–40 ms. The velocity-encoding was set to values of VENC =150–200 cm/s (corresponding to expected peak velocity) for velocity encoding without aliasing, which was 10% above the expected peak blood flow velocity.
Scanned data were acquired under free-breathing conditions using an MR navigator signal read-out for tracking of diaphragmatic motion with a respiratory drift correction of up to a maximum drift 2 mm per minute. The acceptance window of respiratory navigator of 4–8 mm relative to the end-expiratory position was applied to achieve a respiratory acceptance between 40% and 70% and a total acquisition time of 8–12 minutes. A 2.2 mm isovoxel resolution was used to resolve a stack volume with a 25 cm ×25 cm field of view in sagittal orientation and a stack thickness of 6–9 cm. The flip angle was α =10°, TR/TE was 4.7–5.4 ms/2.2–2.8 ms and sensitivity encoding (SENSE) factor for parallel imaging acceleration was 2. A non-symmetric 4-point phase contrast velocity encoding was used. A linear phase correction was applied to velocity-encoded phase contrast data. The slab for 4D-flow MRI covered the aortic arch down to the diaphragm and the pulmonary arteries near the bifurcation (Figures 2,3).
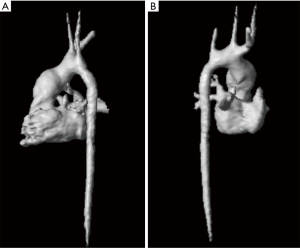
ECG-gated measurement of the short axis stack using a 2D balanced SSFP cinesequence was used to quantify myocardial mass.
Data analysis
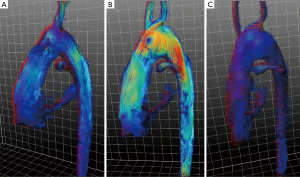
4D-flow MRI data of the HLHS patients and controls were acquired according to common guidelines (6) (Figure 3) and analyzed by an in-house analysis software (MeVisLab-based). Landmarks were set to define the centerline of the aorta between aortic valve and diaphragm (Figure 4A,4B). A curved vessel centerline was defined as the natural cubic spline through the landmarks (Figure 4C). The aorta’s geometric characteristics including curvature and torsion were determined as a function of the position alongside the aortic centerline using the Frenet-Serret formulas. In this context, the term torsion refers to the geometric property of the centerline twist. An in-plane curvature has no such torsion, whereas a curvature which leaves a plane has torsion. The product of torsion and curvature, referred to as ‘effective torsion’, was computed. The aortic arch’s position was determined by the most cephalic point on the centerline (closest to the patient’s head). The sections proximal or distal to this reference point in the aorta are referred to as the ascending or descending neoaorta. The section of the aorta in between the aortic valve and the diaphragm is considered as thoracic aortic section.
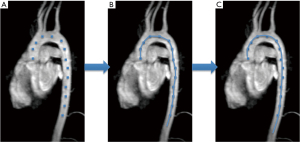
A multi-planar reconstruction (MPR) with slice thickness of 2.2 mm provided blood flow data in planes, perpendicular to the aortic centerline (6). By integration over the MPR planes, helicity density was calculated as a function of the position along the aorta r and time t:
where Vr is the slice volume, u is the velocity vector, ω is the vorticity, and x is the position vector within a plane. A schematic illustration of vorticity and helicity is shown in Figure 5.
Planimetry of the short-axis stack was performed with the Extended MR Workspace (version 2.6.3.5, Philips Healthcare, Best, Netherlands).to quantify myocardial mass. To compare HLHS patients’ peak effective torsion and peak helicity density with the healthy controls’, a Wilcoxon signed rank test was used. Correlations were studied using Spearman’s rank correlation coefficient.
Results
MRI scans were performed in on HLHS subjects (29 male, 13 female) with a median age of 4.9 years (2.9–17.0 years) after establishing the Fontan circulation, and 11 healthy controls (4 male, 7 female) with a mean age of 12.1 (4.0–41.6). The subjects’ demographic characteristics are listed in Table 1.
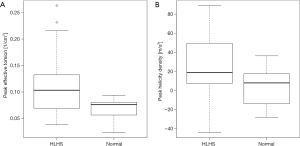
Effective torsion, helicity density of the (neo)aortic root of patients and controls and RV mass are summarized in Table 2. Peak effective torsion was significantly higher in HLHS patients compared to controls (Wilcoxon signed rank test, P=0.02; Figure 6A). In all HLHS subjects, peak effective torsion had a positive sign, corresponding to a path with clockwise rotation in forward direction. Compared to healthy controls, peak helicity density was significantly higher in HLHS patients (Wilcoxon signed rank test, P=0.03; Figure 6B). In 79% of HLHS patients versus 64% of controls, helical blood flow rotated clockwise according to positive peak helicity density values. Both, peak effective torsion and peak helicity density showed large variance for HLHS patients compared to controls reaching particularly large maximum values.
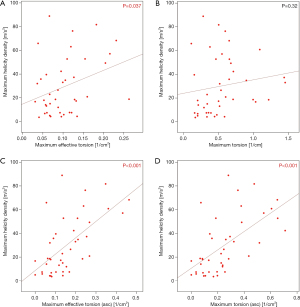
Maximum helicity density of blood flow in the entire aortic section correlated strongly with the maximum effective geometrical torsion of the ascending neoaorta (Spearman’s ρ =0.62, P<0.001) and moderately with the effective torsion of the entire aortic section (Spearman’s ρ =0.35, P=0.02) as shown in Figure 7. Peak helicity density in the entire aortic section correlated moderately with the peak effective torsion of the ascending neoaorta (Spearman’s ρ =0.36, P=0.02). The correlation of the peak helicity density in the entire aortic segment with the effective torsion peak of the entire aortic section was not statistically significant (Spearman’s ρ =0.19, P=0.2).
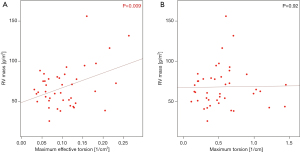
RV myocardial mass was 67.88±25.75 g/m2 BSA and correlated with maximum effective torsion (Spearman’s ρ =0.42, P<0.01), as shown in Figure 8.
Discussion
This study for the first time shows that in HLHS patients with Fontan circulation the abnormally high twist and effective torsion (Figure 2) of the neo-aorta after surgical reconstruction during Norwood operation (Figure 1) induces helical flow patterns (Figures 3,5). The results of our study suggest that patients with strong neoaortic torsion could be at an increased risk to develop hypertrophy.
Aortic twist can be quantified by geometric torsion, a quantity that may reach high values in sections of low curvatures. To mitigate that, we used the effective torsion as a novel index, which considers both torsion and vessel curvature, to characterizes the torsion of the aorta with a focus on regions with high curvature. A previous computational fluid dynamics study suggested that in aortic models, helical flow patterns with dominant preferential direction of rotation in the aorta are related to aortic twist (10). In fluid dynamics, secondary flow patterns with internal stresses, e.g., helical flow patterns, often indicate of energy loss due to resistance to the flow (13). Only few studies have previously investigated ventricular remodeling in HLHS patients and its association to secondary flow patterns induced by the abnormal neoaortic anatomy (3,11,14).
According to previous studies (10,15), curved vascular segments with no significant torsion generate symmetric, anti-directional helices, known as “Dean flow patterns”, with increasing dominance of a particular direction of rotation when torsion increases. By averaging the cross-sectional helicity density, symmetric anti-directional helices with different signs attenuate each other, which makes helicity density an appropriate index to determine the single helix formation or formations with dominant single helical flow patterns.
The significant correlation of neoaorta’s effective torsion with helicity density (Figure 7) and myocardial mass (Figure 8) indicates a consecutive hypertrophy of the right ventricle (RV) to compensate for the increased flow resistance.
Future studies need to investigate whether modifications of aortic arch reconstruction techniques in HLHS, such the chimney reconstruction without patch (16) or arch angle augmentation (17), may change blood flow patterns. Furthermore, it has to be explored if other factors, such as the reduced elasticity of the neo-aorta (5,18) is linked to the formation of abnormal flow pattern. This study may also stimulate others to conduct 4D-flow MRI or CFD studies to help to improve our understanding of aortic biophysical properties after extensive surgical reconstruction.
Conclusions
The abnormal twist of the HLHS patients’ reconstructed neoaorta is associated with unfavorable changes in the aortic fluid dynamics and secondary ventricular hypertrophy. The findings of the present study may ultimately motivate modifications of Norwood procedure.
Acknowledgments
The authors thank Mrs. Traudel Hansen for her support with patient management. We would like to thank cand. med. Eva Rickers for graphic artwork.
Funding: This work was partially supported by an experienced researcher award from the Alexander von Humboldt Foundation to Prof. Kheradvar.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yskert von Kodolitsch, Harald Kaemmerer, Koichiro Niwa) for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part IV” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-770
Data Sharing Statement: Available at http://dx.doi.org/10.21037/cdt-20-770
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-770). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part IV” was commissioned by the editorial office without any funding or sponsorship. Dr. Kheradvar reports grants from Humboldt Foundation, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Kiel University’s Faculty of Medicine (A168/07). The patient’s ascent and the parent’s consent were obtained prior to any MRI study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med 1983;308:23-6. [Crossref] [PubMed]
- Chang AC, Farrell PE, Murdison KA, et al. Hypoplastic left heart syndrome: hemodynamic and angiographic assessment after initial reconstructive surgery and relevance to modified Fontan procedure. J Am Coll Cardiol 1991;17:1143-9. [Crossref] [PubMed]
- Schäfer M, DiMaria MV, Jaggers J, et al. Suboptimal neoaortic arch geometry correlates with inefficient flow patterns in hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2019;158:e113-6. [Crossref] [PubMed]
- Haller C, Chetan D, Saedi A, et al. Geometry and growth of the reconstructed aorta in patients with hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg 2017;153:1479-87.e1. [Crossref] [PubMed]
- Voges I, Jerosch-Herold M, Hedderich J, et al. Maladaptive aortic properties in children after palliation of hypoplastic left heart syndrome assessed by cardiovascular magnetic resonance imaging. Circulation 2010;122:1068-76. [Crossref] [PubMed]
- Dyverfeldt P, Bissell M, Barker AJ, et al. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson 2015;17:72-90. [Crossref] [PubMed]
- Bollache E, van Ooij P, Powell A, et al. Comparison of 4D flow and 2D velocity-encoded phase contrast MRI sequences for the evaluation of aortic hemodynamics. Int J Cardiovasc Imaging 2016;32:1529-41. [Crossref] [PubMed]
- Voges I, Scheewe J, Attmann T, et al. Abnormal aortic arch shape and vortical flow patterns are associated with descending aortic dilatation in patients with hypoplastic left heart syndrome. Int J Cardiol 2020:S0167-5273(20)33842-0.
- Tse KM, Chang R, Lee HP, et al. A computational fluid dynamics study on geometrical influence of the aorta on haemodynamics. Eur J Cardiothorac Surg 2013;43:829-38. [Crossref] [PubMed]
- Liu X, Fan Y, Deng X, et al. Effect of Spiral Flow on Transport of Oxygen in the Aorta: A Numerical Study. Ann Biomed Eng 2010;38:917-26. [Crossref] [PubMed]
- Bruse JL, Cervi E, McLeod KModeling of Congenital Hearts Alliance (MOCHA) Collaborative Group, et al. Looks Do Matter! Aortic Arch Shape After Hypoplastic Left Heart Syndrome Palliation Correlates With Cavopulmonary Outcomes. Ann Thorac Surg 2017;103:645-54. [Crossref] [PubMed]
- Bove EL, Mosca RS. Surgical repair of the hypoplastic left heart syndrome. Hypoplastic Left Heart Syndrome 1996;5:23-35.
- Kheradvar A, Pedrizzetti G. Vortex Formation in the Cardiovascular System. Springer, 2012.
- Biglino G, Giardini A, Ntsinjana HN, et al. Ventriculoarterial coupling in palliated hypoplastic left heart syndrome: noninvasive assessment of the effects of surgical arch reconstruction and shunt type. J Thorac Cardiovasc Surg 2014;148:1526-33. [Crossref] [PubMed]
- Yamamoto K, Aribowo A, Hayamizu Y, et al. Visualization of the flow in a helical pipe. Fluid Dyn Res 2002;30: [Crossref]
- Asada S, Yamagishi M, Itatani K, et al. Chimney reconstruction of the aortic arch in the Norwood procedure. J Thorac Cardiovasc Surg 2017;154:e51-4. [Crossref] [PubMed]
- Hasegawa T, Yoshihiro O, Ayako M, et al. Aortic arch geometry after the Norwood procedure: The value of arch angle augmentation. J Thorac Cardiovasc Surg 2015;150:358-66. [Crossref] [PubMed]
- Logoteta J, Ruppel C, Hansen JH, et al. Ventricular function and ventriculo-arterial coupling after palliation of hypoplastic left heart syndrome: A comparative study with Fontan patients with LV morphology. Int J Cardiol 2017;227:691-7. [Crossref] [PubMed]


