
Systemic sclerosis-associated pulmonary arterial hypertension in children
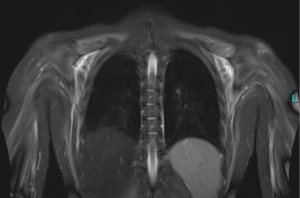
Systemic sclerosis (SSc) usually presents with a combination of vasculopathy, inflammation, autoimmunity, and fibrogenesis that can individually vary (Figures 1-3) (1). Major complications can include digital vasculopathy, gastrointestinal complications, cardiac and pulmonary fibrosis, scleroderma renal crisis, digital contractures, calcinosis, and acro-osteolysis (2).
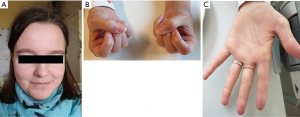

The etiology and pathogenesis of SSc is largely unknown. There is evidence of multifactorial genesis with interacting genetic, vascular, autoimmunological and metabolic factors (3,4). Genome-wide analysis showed a significant association of SSc with modifications of the fibrillin-1 gene and other genes involved in collagen metabolism. In skin and lung fibroblasts, collagen production is activated by transcription. Other candidate genes relate to HLA molecules and inflammation mediators, especially transforming growth factor β (TGF-β) and connective tissue growth factor (CTGF). Furthermore, a disturbed expression of metalloproteinases and their inhibitors has been described. An increased expression of cytokines and chemokines (IL-1, IL-4, TNF-α, IL-17, IL-21, MCP-1, MCP-2) can lead to an increased presence of immunological effector cells in the target organ. Lymphocytes may be sensitized to components of the connective tissue and stimulate fibroblasts to increase collagen production. Further evidence of autoimmunity in SSc results from the detection of antinuclear antibodies and an association with HLA class I and II antigens (HLA-B8, −DR3, −DR5) (4).
SSc is rare in children with a reported annual incidence of 0.27 to 0.5 per million children age <16 years (5,6), and a prevalence of 3 per million (7,8). Characteristic skin changes were the most common feature reported in the largest series of 153 patients with juvenile SSc, followed by Raynaud’s phenomenon, musculoskeletal and cardiopulmonary symptoms (9). Approximately 10% of adult patients with SSc are affected by pulmonary arterial hypertension (PAH). Corresponding data in children with SSc is rare, however PAH appears to be similar frequently in juvenile and adult SSc (4,9-12).
In adult patients, PAH associated with connective tissue disease (CTD-PAH), including SSc, is the most commonly identified type of disease-associated PAH (13). According to data of the largest pediatric PH registry, the global TOPP-registry (Tracking Outcomes and Practice in Pediatric Pulmonary Hypertension), CTD-PAH affected 3% of 362 registered children with invasively confirmed pulmonary hypertension (14).
Pulmonary arterial hypertension in SSc
PAH is a progressive disease of the pulmonary vessels that eventually leads to right ventricular failure and death if left untreated. PAH is a serious complication of many connective tissue diseases (CTD), including SSc, systemic lupus erythematosus, mixed CTD, and, to a lesser extent, dermatomyositis, and Sjögren’s syndrome (15).
The prognosis of CTD-PAH patients is affected by the underlying CTD etiology and disease severity, but it is known from adult data, that patients with SSc-PAH might have poor prognosis (16,17). While children with SSc usually have better outcome compared to adult SSc due to lower frequency of severe organ involvement (18,19), PAH is still one of the leading causes of morbidity and mortality in patients with SSc (19-22).
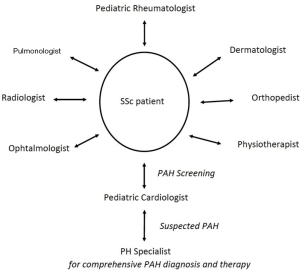
A lack of awareness of SSc-PAH related symptoms among treating physicians and patients can cause delays in diagnosis and therapy. However, while SSc-PAH can progress rapidly, early detection and treatment is essential (23,24). Therefore, regular screening for PAH of SSc patients in an interdisciplinary setting is considered to play an important role in the disease management (Figure 4). In adults, annual PAH-screening of SSc patients is recommended (15), and there is growing evidence to support this approach also in pediatric SSc patients.
Patients with SSc-PAH frequently present with non-specific symptoms, therefore it is reasonable to have a high index of suspicion in this at-risk population. Red-flag findings for the presence of SSc-PAH should optimally be recognized and seeked for, such as decreased diffusing capacity of the lung for carbon monoxide (DLCO), increased ratio of forced vital capacity to DLCO, and an abnormal antinuclear antibody pattern (25). In contrast to adult SSc-PAH, presence of anti-centromere antibodies does not seem to be appropriate for PAH screening in children with SSc, as they can be found only rarely in childhood, and no correlation could be detected between anti-centromere antibodies and the manifestation of PAH in juvenile SSc (18,26).
Transthoracic echocardiography is a recommended assessment tool for PAH-screening of patients with SSc (15). A tricuspid valve regurgitation Doppler velocity >2.8 m/sec is regarded as suspicious of the presence of PAH. If no tricuspid valve regurgitation is available, indirect markers of evidence for possible PAH include right ventricular dilatation, right ventricular dysfunction, systolic flattening of the interventricular septum, and Doppler abnormalities of pulmonary arterial blood flow (increased acceleration time, systolic notch).
Additional screening options include pulmonary function tests, exercise stress testing, and measurement of cardiac biomarkers, i.e., brain natriuretic peptide (15,27).
Composite screening algorithms can increase the sensitivity and negative predictability of testing compared to single screening methods alone.
Importance of a systematic diagnostic approach
It has to be considered that pulmonary hypertension in SSc can present with varying features of precapillary (pulmonary vascular disease), intrapulmonary (due to interstitial lung disease) and postcapillary (as a consequence of left ventricular myocardial dysfunction) disease.
A precise differential diagnosis of PAH is often challenging (15,28), but careful phenotyping of PAH in SSc patients, usually including systematic invasive evaluation by cardiac catheterization, is critical to allow prognostic assessment and targeted treatment (29,30).
PH due to interstitial lung disease (ILD, group 3 PH) often has worse prognosis than SSc-PAH (31). PAH-specific therapies have not shown to improve symptoms and outcome in ILD, and may even cause an increased risk of hypoxia in SSc patients with ILD and PH (30).
Post-capillary PH can be due to myocardial fibrosis and resulting left ventricular dysfunction (group 2 PH) (30). There is no clear evidence that PAH-specific therapies are able to improve outcome in SSc patients with left heart disease (32). Additional pulmonary veno-occlusive components can also complicate SSc-PAH, and are associated with worse survival and increased risk of pulmonary edema if PAH therapies are initiated.
Therefore, relevant interstitial lung disease and left heart disease should be ruled out in the differential diagnosis of SSc-PAH before starting any targeted therapy.
Treatment of SSc-PAH
The following targeted drugs are meanwhile available for specific therapy of PAH addressing three pathophysiologic pathways (nitric oxide, endothelin, and prostacyclin pathways): the phosphodiesterase-5 (PDE-5) inhibitors (Sildenafil and Tadalafil); Riociguat, a soluble guanylate cyclase stimulator; the endothelin receptor antagonists (ERA) (ambrisentan, bosentan and macitentan), the prostacyclin analogues iloprost (inhalation), epoprostenol, treprostinil (subcutaneous and intravenous), and the prostacyclin (IP) receptor agonist Selexipag (33,34). Owing to the rarity of the disease in children, and the lack of randomized controlled clinical studies, the majority of these drugs is used off-label, only Sildenafil and Bosentan are currently approved by the European regulatory agency (EMA) for specific therapy of children with PAH beyond infancy (33,34).
Due to the progressive character of SSc-PAH known from adult studies, it appears appropriate to initiate targeted PAH-therapy in juvenile SSc-PAH early. If left untreated, the prognosis for SSc-PAH might be quite poor with reasonable mortality, similar to other progressive forms of PAH (i.e., idiopathic or heritable PAH).
Recently, adult registries have demonstrated, that the emergence of new treatments and use of combination therapy has improved survival for SSc-PAH patients (32). Both, the 2015 European Society of Cardiology/European Respiratory Society (ESC/ERS) and 2017 European League Against Rheumatism (EULAR) guidelines therefore recommend that adult patients with SSc-PAH should be treated according to evidence-based treatment algorithms (32,35). Growing evidence has established initial double combination therapy as a key strategy for treating adult patients with PAH, including those with SSc-PAH. The 6th WSPH proceedings recommend initial monotherapy only in a minority of PAH patients (32). A structured follow-up, particularly early after treatment initiation, should allow for the identification of early signs of disease progression to intensify treatment. Recently, corresponding recommendations for PAH in children adapted from adult treatment algorithms became available (36,37). Dual combination therapy regimens (i.e., ERA and a PDE-5 inhibitor) are increasingly used for patients with a low- or intermediate-risk status (36), and there is growing evidence to support this approach in CTD-PAH patients (38,39).
In SSc-PAH, the targeted PAH-therapy is usually combined with immunomodulatory/immunosuppressive drugs to control the inflammatory process and slow the progression towards fibrosis (i.e., cyclophosphamide, mycophenolate mofetil) (40).
Concluding remarks
PAH is a serious complication in children with SSc associated with a high risk to deteriorate and increased morbidity and mortality. Therefore, treating physicians must be aware of the association between SSc and PAH, and interdisciplinary care of SSc patients is essential. Regular screening by pediatric cardiologists may enable early diagnosis, which should be followed by structured diagnostic testing and comprehensive PAH-targeted treatment by PH specialists.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Cardiovascular Diagnosis and Therapy for the series “Pediatric Pulmonary Hypertension”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-901). The series “Pediatric Pulmonary Hypertension” was commissioned by the editorial office without any funding or sponsorship. Dr. Apitz served as the unpaid Guest Editor of the series. Dr. Apitz reports personal fees from Actelion, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hedrich CM, Fiebig B, Hahn G, et al. Presentations and treatment of childhood scleroderma: localized scleroderma, eosinophilic fasciitis, systemic sclerosis, and graft-versus-host disease. Clin Pediatr (Phila) 2011;50:604-14. [Crossref] [PubMed]
- Murray KJ, Laxer RM. Scleroderma in children and adolescents. Rheum Dis Clin North Am 2002;28:603-24. [Crossref] [PubMed]
- Rabinovich CE. Challenges in the diagnosis and treatment of juvenile systemic sclerosis. Nat Rev Rheumatol 2011;7:676-80. [Crossref] [PubMed]
- Stevens AM, Torok KS, Li SC, et al. Immunopathogenesis of Juvenile Systemic Sclerosis. Front Immunol 2019;10:1352. [Crossref] [PubMed]
- Herrick AL, Ennis H, Bhushan M, et al. Incidence of childhood linear scleroderma and systemic sclerosis in the UK and Ireland. Arthritis Care Res (Hoboken) 2010;62:213-8. [Crossref] [PubMed]
- Pelkonen PM, Jalanko HJ, Lantto RK, et al. Incidence of systemic connective tissue diseases in children: a nationwide prospective study in Finland. J Rheumatol 1994;21:2143-6. [PubMed]
- Royle JG, Lanyon PC, Grainge MJ, et al. The incidence, prevalence, and survival of systemic sclerosis in the UK Clinical Practice Research Datalink. Clin Rheumatol 2018;37:2103-11. [Crossref] [PubMed]
- Beukelman T, Xie F, Foeldvari I. Assessing the prevalence of juvenile systemic sclerosis in childhood using administrative claims data from the United States. J Scler Relat Disord 2018;3:189-90. [Crossref]
- Martini G, Foeldvari I, Russo R, et al. Juvenile scleroderma working group of the Pediatric Rheumatology European Society. Systemic sclerosis in childhood: clinical and immunologic features of 153 patients in an international database. Arthritis Rheum 2006;54:3971-8. [Crossref] [PubMed]
- Adrovic A, Oztunc F, Barut K, et al. The frequency of pulmonary hypertension in patients with juvenile scleroderma. Bosn J Basic Med Sci 2015;15:30-5. [Crossref] [PubMed]
- Foeldvari I, Zhavania M, Birdi N, et al. Favourable outcome in 135 children with juvenile systemic sclerosis: results of a multi-national survey. Rheumatology 2000;39:556-9. [Crossref] [PubMed]
- Foeldvari I, Nihtyanova SI, Wierk A, et al. Characteristics of patients with juvenile onset systemic sclerosis in an adult single-center cohort. J Rheumatol 2010;37:2422-6. [Crossref] [PubMed]
- Yang X, Mardekian J, Sanders KN, et al. Prevalence of pulmonary arterial hypertension in patients with connective tissue diseases: a systematic review of the literature. Clin Rheumatol 2013;32:1519-31. [Crossref] [PubMed]
- Berger RM, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet 2012;379:537-46. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JLESC Scientific Document Group, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Chung L, Farber HW, Benza R, et al. Unique predictors of mortality in patients with pulmonary arterial hypertension associated with systemic sclerosis in the REVEAL registry. Chest 2014;146:1494-504. [Crossref] [PubMed]
- Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017;50:1700740 [Crossref] [PubMed]
- Scalapino K, Arkachaisri T, Lucas M, et al. Childhood onset systemic sclerosis: classification, clinical and serologic features, and survival in comparison with adult onset disease. J Rheumatol 2006;33:1004-13. [PubMed]
- Martini G, Vittadello F, Kasapçopur O, et al. Factors affecting survival in juvenile systemic sclerosis. Rheumatology 2009;48:119-22. [Crossref] [PubMed]
- Quartier P, Bonnet D, Fournet JC, et al. Severe cardiac involvement in children with systemic sclerosis and myositis. J Rheumatol 2002;29:1767-73. [PubMed]
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis 2007;66:940-4. [Crossref] [PubMed]
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809-15. [Crossref] [PubMed]
- Coghlan JG, Wolf M, Distler O, et al. Incidence of pulmonary hypertension and determining factors in patients with systemic sclerosis. Eur Respir J 2018;51:1701197 [Crossref] [PubMed]
- Mihai C, Antic M, Dobrota R, et al. Factors associated with disease progression in early-diagnosed pulmonary arterial hypertension associated with systemic sclerosis: longitudinal data from the DETECT cohort. Ann Rheum Dis 2018;77:128-32. [Crossref] [PubMed]
- Denton CP, Khanna D. Systemic sclerosis. Lancet 2017;390:1685-99. [Crossref] [PubMed]
- Foeldvari I, Tyndall A, Zulian F, et al. Juvenile and young adult-onset systemic sclerosis share the same organ involvement in adulthood: data from the EUSTAR database. Rheumatology 2012;51:1832-7. [Crossref] [PubMed]
- Allanore Y, Borderie D, Avouac J, et al. High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Arthritis Rheum 2008;58:284-91. [Crossref] [PubMed]
- Lammers AE, Apitz C, Zartner P, et al. Diagnostics, monitoring and outpatient care in children with suspected pulmonary hypertension/paediatric pulmonary hypertensive disease. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016;102:ii1-ii13. [Crossref] [PubMed]
- Apitz C, Hansmann G, Schranz D. Haemodynamic assessment and acute vasoreactivity testing in the evaluation of children with pulmonary vascular disease. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016;102:ii23-ii29. [Crossref] [PubMed]
- Launay D, Sobanski V, Hachulla E, et al. Pulmonary hypertension in systemic sclerosis: different phenotypes. Eur Respir Rev 2017;26:170056 [Crossref] [PubMed]
- Lefèvre G, Dauchet L, Hachulla E, et al. Survival and prognostic factors in systemic sclerosis-associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis Rheum 2013;65:2412-23. [Crossref] [PubMed]
- Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019;53:1801889 [Crossref] [PubMed]
- Latus H, Schranz D, Apitz C. Treatment of pulmonary arterial hypertension in children. Nat Rev Cardiol 2015;12:244-54. [Crossref] [PubMed]
- Hansmann G, Apitz C, Humpl T, et al. Urgent need for off-label use of PAH medications and reimbursement for children with pulmonary hypertension: Statement of the Working Group on Pulmonary Hypertension of the German Society for Pediatric Cardiology and Congenital Cardiac Defects (DGPK). Monatsschr Kinderheilkd 2020;168:733-8. [Crossref]
- Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017;76:1327-39. [Crossref] [PubMed]
- Hansmann G, Koestenberger M, Alastalo T, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension. The European Pediatric pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant 2019;38:879-901. [Crossref] [PubMed]
- Rosenzweig EB, Abman SH, Adatia I, et al. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J 2019;53:1801916 [Crossref] [PubMed]
- Coghlan JG, Galiè N, Barberà JAAMBITION investigators, et al. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis 2017;76:1219-27. [Crossref] [PubMed]
- Pan J, Lei L, Zhao C. Comparison between the efficacy of combination therapy and monotherapy in connective tissue disease associated pulmonary arterial hypertension: a systematic review and meta-analysis. Clin Exp Rheumatol 2018;36:1095-102. [PubMed]
- Zloof Y, Schonfeld T, Dagan T, et al. Systemic sclerosis sine scleroderma with pulmonary arterial hypertension in a 3-year-old girl. Pediatrics 2020;145:e20192504 [Crossref] [PubMed]


