
Infective endocarditis and oral health—a Narrative Review
Introduction
Infective endocarditis (IE) is an uncommon but usually severe and often fatal inflammatory disease of the endocardium. The potentially prominent role of oral pathogens in the development of endocarditis, which enter the bloodstream as part of physiological processes, but especially during certain dental procedures, has been emphasized for decades (1). Considering increasing antimicrobial resistance, especially the effectiveness of a prophylactic antibiotic regimen prior to dental interventions to prevent possible endocarditis has been controversially discussed time and again.
Particularly within the last five years, numerous professional societies worldwide have published guidelines and updates on the prevention of endocarditis, revealing partly very divergent interpretations of the available evidence. The different recommendations derived from this has unavoidably led to a confusing and contradictory recommendation situation for patients with underlying cardiac conditions and clinicians alike (2-4). A critical analysis of the published recommendations and their evaluation in the current state of science is therefore essential. This review was based on a systematic search for the relevant literature conducted in MEDLINE/Pubmed including articles Epub ahead of print. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-908).
Infective endocarditis
The development of endocarditis requires a complex interplay of different factors that cause certain microorganisms to adhere to the cardiac endothelium and trigger an inflammatory process (1,5). Congenital or acquired diseases of the heart can lead to partial loss of endothelial integrity, resulting in an increased risk for the development of infective endocarditis (5-7). Loss of endothelial integrity and disruption of the inner cell layer enable the formation of a platelet-derived thrombus and a corresponding inflammatory cascade. The resulting non-bacterial endocarditis (NBTE) is considered a prerequisite for the adhesion of microorganisms that enter the bloodstream in the course of bacteremia (1,5,8).
The cause of transient bacteremia usually lies in the disintegration of a bacterially colonized surface, such as the skin or mucous membrane, which harbors numerous commensals but also opportunistic pathogens. While up to 90% of IE are caused by gram-positive Staphylococcus sp. (species), Streptococcus sp. and Enterococcus sp. (9), Staphylococcus aureus is considered the most common in high-income countries (10). Coagulase-negative Staphylococcus sp. such as S. epidermis are ubiquitous skin commensals and account for approximately 10% of IE whereas streptococcal infections by the oral viridans group (e.g., S. mutans, S. mitis, S. anguinis) cause almost 20% of infective endocarditis (9). Hence, a more precise evaluation of the oral cavity as an outstanding environment for bacteria is reasonable. The oral microbiome consists of over 700 different species of bacteria (11), including numerous commensal and also opportunistic pathogens, some of which are organized in complex biofilms entering the bloodstream in the event of mucosal or gingival disruption. Traumatic injury to the mucosa or gingiva as part of physiological processes such as chewing food or oral hygiene (12), but also particularly in diseases such as dental caries or periodontitis and their secondary symptoms, as well as in various therapeutic measures for their prevention and treatment, can thus inevitably lead to the transfer of bacterial pathogens from the oral cavity into the bloodstream, resulting in bacteremia (1). Bacterial adhesion of microorganisms to disrupted endothelial walls and resulting thrombotic formations is promoted by specific mediators such as surface components that serve as virulence factors in various microbial species (1,13-16). In this manner, several species of streptococci, staphylococci and enterococci can colonize impaired endocardium and cause infective endocarditis.
Dental conditions and oral bacteria
Dental caries and periapical conditions
Dental caries is considered an infectious disease mediated by a pathogenic bacterial biofilm that may form on tooth surfaces (17). A change of the microbial composition to a more acidic environment favors proliferation and phenotypic adaptation of acid-tolerant bacteria inducing a dysbiotic shift of the biofilm. Dsybiosis refers to changes of the relative amount of certain bacteria ultimately resulting in the transition from a commensalic to a highly pathogenic bacterial community. In terms of dental caries this shift eventually enhances the acidic potential of the biofilm and thus leads to demineralization of dental hard tissues known as caries (18,19).
While initially it was assumed that Lactobacillus sp. and Streptococcus sp, especially Streptococcus mutans, were responsible for the acidification of the biofilm (20,21), more recently composed etiological models have shown that many other endogenous bacteria found in the dental biofilm are capable of acidification. Others are able to increase pH values and thus counteract over-acidification (21-23).
Although the formation of a dental biofilm is known to be essential for the initial development of carious lesions, it must be emphasized that dental caries is based on a complex multifactorial etiology in which, in addition to the biofilm, interactions between components such as salivary flow and composition, structure of dental hard tissues, environmental factors and genetic predisposition are required (24).
Behavioral factors such as dietary habits and oral hygiene play a crucial role in the formation of carious lesions. Continuous intake of fermentable carbohydrates, i.e., sugar leads to a prolonged reduction of pH value in the biofilm (17), which causes the demineralization of hydroxyapatite (25,26), ultimately resulting in manifestation of a clinically detectable caries lesion, including discoloration and cavitation (27) (Figure 1).
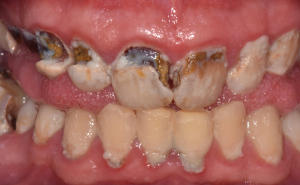
As the carious lesion progresses, it increasingly destroys parts of the clinical crown. If left untreated, the lesion reaches the pulp chamber and causes an initially reversible and then irreversible inflammation, which inevitably leads to necrosis of the entire pulp soft tissue.
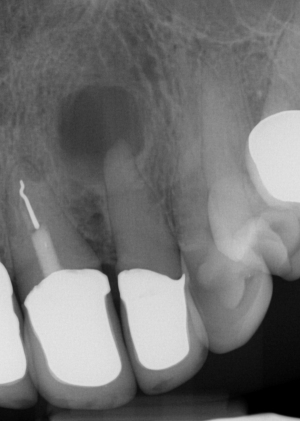
Consequently, bacteria initially colonize the necrotic tissue and then penetrate the periapical tissue, i.e., the apical parts of the periodontal ligament and the surrounding alveolar bone (Figure 2). In these inflammatory lesions, there is usually a comparatively diverse and complex bacterial flora consisting of 10–20 different, predominantly Gram-negative species (28,29). In contrast, in the event of recurrence in teeth already endodontically treated, there is a reduced bacterial spectrum consisting of 3-6 different gram-positive and gram-negative species, including Staphylococcus sp. (30). The described bacterial infection usually leads to an inflammatory process within the periapical tissue that often has a chronic, asymptomatic course. The absence of clinical symptoms such as pain or swelling usually leads to the inflammatory process manifesting itself over a longer period, remaining undetected.
Periodontal disease
Besides dental caries and its consequences, diseases of the tooth surrounding and supporting tissues are highly prevalent and must be mentioned in this context. Periodontitis is a multifactorial disease that is associated with dysbiotic dental biofilms and is considered one of the most common chronic inflammatory disease in humans (31). Migration of dental biofilm from the gingival margin into the gingival crevice lead to a shift of the microbial community, consisting of approximately 100–300 endogenous microorganisms, from a symbiotic to a dysbiotic state, resulting in the formation of a periodontal pocket and further shifts in bacterial composition (32-34). The formation of a periodontal pocket is accompanied by irreversible loss of tooth supporting soft and hard tissues, ultimately resulting in tooth loss (Figure 3). For a long period of time, the prevailing hypothesis was that several specific gram-negative anaerobic bacteria such as Prophyromonas gingivalis, Tannerella forsythia, Treponema denticola and Aggregatibacter actinomycetemcomitans are major determinants of the pathogenesis of periodontitis (35). However, recent results of metagenomics analysis have shown that the relative abundance of many different bacterial species may significantly influence the pathogenicity of the periodontal biofilm, consequently leading to an altered, excessive host immune response. Thus, it is not so much individual bacterial species, but rather the mutual complex dysbiotic shift of the biofilm and the increased release of inflammatory cytokines and degenerative enzymes that ultimately lead to the destruction of periodontal soft and hard tissues (32).
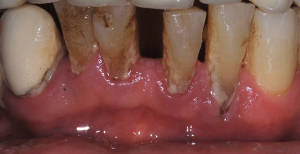
Dental conditions causing bacteremia
As the described odontogenic infections are associated with pathogenic microorganisms, gingival or mucosal trauma or the manipulation of the periapical region of the tooth may cause a transient bacteremia of these microorganisms that, in a patient at risk, can cause infective endocarditis. Iatrogenic gingival or mucosal traumas mainly occur during dental procedures such as tooth extractions, periodontal and apical surgery, removal of caries affecting cervical or subgingival portions of the tooth, dental prophylaxis as well as non-surgical periodontal therapy (36). Endodontic treatment on the other hand may lead to manipulation of the periapical region, causing bacteremia of microorganisms involved in endodontic and periapical inflammation (37). In addition, diagnostic procedures like periodontal probing may cause micro trauma in the apical lining of a periodontal pocket, resulting in clinically visible gingival bleeding. Besides gingival and mucosal trauma that occur during dental procedures, clinical studies haven proven oral bacteremia after daily activities such as chewing, tooth brushing and flossing (38). Since, it is commonly accepted that the presence and the magnitude of bacteremia caused by odontogenic infections are directly proportional to the severity of oral inflammation (39,40), good oral and dental health has to be highlighted as a major factor regarding prevention of infective endocarditis (3,12,36,41,42).
Current strategies on prevention
The entity of infective endocarditis was first described over 130 years ago and has been the subject of research ever since. In the 1950s, the American Heart Association (AHA) have released the first clinical guideline for the prevention of IE highlighting dental procedures and antibiotic prophylaxis (43). Since then, the AHA (36,44-50), the European Society of Cardiology (ESC) (3,14,51) and several other national and multinational societies have published guidelines and regular updates for the prevention of infective endocarditis with various recommendations regarding the requirement of antibiotic prophylaxis before dental procedures (Table 1). Especially considering patients suffering from congenital heart defects (CHD), however, there is still no uniform opinion on whether these recommendations on prevention can be fully applied (52,53). Always considering the patient’s cardiac risk and the growing development of antibiotic resistance in the society, the regularly updated AHA and ESC guidelines have increasingly tightened the indication for antibiotic prophylaxis in recent years and currently only recommend it for patients with the highest risk of developing endocarditis (3,42). In contrast to these internationally acknowledged recommendations, the British National Institute for Health and Care Excellence (NICE), under the leadership of the National Health Service (NHS), decided in 2008 to withdraw all recommendations for antibiotic prophylaxis prior to dental procedures, regardless of the patient's individual cardiac risk profile or the type of dental procedure (54).
Table 1
| Society | Year | Patient risk profile - assumed cardiac conditions with increased susceptibility for IE | Procedure risk profile - dental procedures requiring antibiotic prophylaxis | Recommended oral antibiotic regimen |
|---|---|---|---|---|
| AHA | 1997 | High (*) and moderate risk for infective endocarditis: | 1. Dental extractions | Amoxicillin 2 g, clindamycin 600 mg/Azithromycin/Clarithromycin 500 mg, 60 minutes pre-operative |
| 1. Prosthetic cardiac valves, including bioprosthetic and homograft valves* | 2. Periodontal procedures including surgery, scaling and root planning, probing and recall maintenance | |||
| 2. Previous bacterial endocarditis* | 3. Dental implant placement and reimplantation of avulsed teeth | |||
| 3. Complex cyanotic congenital heart disease (e.g., single ventricle states, transposition of the great arteries, tetralogy of Fallot)* | 4. Endodontic (root canal) instrumentation or surgery only beyond the apex | |||
| 4. Surgically constructed systemic pulmonary shunts or conduits* | 5. Subgingival placement of antibiotic fibers or strips | |||
| 5. Most other congenital cardiac malformations | 6. Initial placement of orthodontic bands but not brackets | |||
| 6. Acquired valvar dysfunction (e.g., rheumatic heart disease) | 7. Intraligamentary local anesthetic injections | |||
| 7. Hypertrophic cardiomyopathy | 8. Prophylactic cleaning of teeth or implants where bleeding is anticipated | |||
| 8. Mitral valve prolapse with valvar regurgitation and/or thickened leaflets | ||||
| ESC | 2004 | High (*) and moderate risk for infective endocarditis: | Dental procedures with the risk of gingival/mucosal trauma | Amoxicillin 2 g, clindamycin 600 mg, Azithromycin/Clarithromycin 500 mg, 60 minutes pre-operative |
| 1. Prosthetic heart valves* | ||||
| 2. Complex congenital cyanotic heart diseases* | ||||
| 3. Previous infective endocarditis* | ||||
| 4. Surgically constructed systemic or pulmonary conduits* | ||||
| 5. Acquired valvular heart diseases | ||||
| 6. Mitral valve prolapse with valvular regurgitation or severe valve thickening | ||||
| 7. Non-cyanotic congenital heart diseases (except for secundum type ASD) including bicuspid aortic valves | ||||
| 8. Hypertrophic cardiomyopathy | ||||
| AHA | 2007 | High risk for infective endocarditis: | Dental procedures that involve manipulation of gingival tissue or the periapical region of teeth or perforation of the oral mucosa | Amoxicillin 2 g, Cephalexin 2 g/Clindamycin 600 mg, Azithromycin/Clarithromycin 500 mg, 30–60 minutes pre-operative |
| 1. Prosthetic cardiac valve or prosthetic material used for cardiac valve repair | ||||
| 2. Previous infective endocarditis | ||||
| 3. Congenital heart disease (CHD)* | ||||
| - Unrepaired cyanotic CHD, including palliative shunts and conduits | ||||
| - Completely repaired congenital heart defect with prosthetic material or | ||||
| device, whether placed by surgery or by catheter intervention, during the first | ||||
| six months after the procedure† | ||||
| - Repaired CHD with residual defects at the site or adjacent to the site of a | ||||
| prosthetic patch or prosthetic device (which inhibit endothelialization) | ||||
| 4. Cardiac transplantation recipients who develop cardiac valvulopathy | ||||
| NICE | 2008 | Increased risk for infective endocarditis | None | None |
| 1. acquired valvular heart disease with stenosis or regurgitation | ||||
| 2. hypertrophic cardiomyopathy | ||||
| 3. previous infective endocarditis | ||||
| 4. structural congenital heart disease, including surgically corrected or palliated structural conditions, but excluding isolated atrial septal defect, fully repaired ventricular septal defect or fully repaired patent ductus arteriosus, and closure devices that are judged to be endothelialised | ||||
| ESC | 2009 | Only highest risk for infective endocarditis: | Dental procedures requiring manipulation of the gingival or periapical region of the teeth or perforation of the oral mucosa not including local anesthetic injections in non-infected tissues, treatment of superficial caries, removal of sutures, dental X-rays, placement or adjustment of removable prosthodontic or orthodontic appliances or braces. Prophylaxis is also not recommended or following the shedding of deciduous teeth or trauma to the lips and oral mucosa | Amoxicillin 2 g |
| 1. Patients with a prosthetic valve or a prosthetic material used for cardiac valve repair | Clindamycin 600 mg, 30–60 minutes pre-operative | |||
| 2. Patients with previous IE | ||||
| 3. Patients with CHD: | ||||
| - cyanotic CHD, without surgical repair, or with residual defects, palliative shunts or conduits | ||||
| - CHD with complete repair with a prosthetic material, whether placed by surgery or by percutaneous techniques, up to 6 months after the procedure | ||||
| - when a residual defect persists at the site of implantation of a prosthetic material or device by cardiac surgery or percut technique | ||||
| NICE | 2015 | 1. acquired valvular heart disease with stenosis or regurgitation | None | None |
| 2. hypertrophic cardiomyopathy | ||||
| 3. previous infective endocarditis | ||||
| 4. structural congenital heart disease, including surgically corrected or palliated structural conditions, but excluding isolated atrial septal defect, fully repaired ventricular septal defect or fully repaired patent ductus arteriosus, and closure devices that are judged to be endothelialised | ||||
| 5. valve replacement | ||||
| ESC | 2015 | High risk for infective endocarditis: | Dental procedures requiring manipulation of the gingival or periapical region of the teeth or perforation of the oral mucosa, not including local anesthetic injections in non-infected tissues, treatment of superficial caries, removal of sutures, dental X-rays, placement or adjustment of removable prosthodontic or orthodontic appliances or braces or following the shedding of deciduous teeth or trauma to the lips and oral mucosa | Amoxicillin 2 g, Clindamycin 600 mg, 30–60 minutes pre-operative |
| 1. Patients with any prosthetic valve, including a transcatheter valve, or those in whom any prosthetic material was used for cardiac valve repair. | ||||
| 2. Patients with a previous episode of IE. | ||||
| 3. Patients with CHD: | ||||
| - Any type of cyanotic CHD. | ||||
| - Any type of CHD repaired with a prosthetic material, whether placed surgically or by percutaneous techniques, up to 6 months after the procedure or lifelong if residual shunt or valvular regurgitation remains. | ||||
| BDA | 2015 | High risk for infective endocarditis: | Dental procedures requiring manipulation of the gingival (including extractions and scaling) or periapical region of the teeth (including root canal procedures) or perforation of the oral mucosa | Amoxicillin 2 g, Clindamycin 600 mg, 30–60 minutes pre-operative |
| 1. Patients with a previous history of infective endocarditis | ||||
| 2. Patients with any form of prosthetic heart valve (including a transcatheter valve) | ||||
| 3. Those in whom prosthetic material was used for cardiac valve repair | ||||
| 4. Patients with any type of cyanotic congenital heart disease | ||||
| 5. Patients with any type of congenital heart disease repaired with prosthetic material, whether placed surgically or by percutaneous techniques, for the first 6 months after the procedure or lifelong if a residual shunt or valvular regurgitation remains | ||||
| AHA | 2017 | High risk for infective endocarditis: | Dental procedures that involve manipulation of gingival tissue or the periapical region of teeth or perforation of the oral mucosa | Amoxicillin 2 g, Cephalexin 2 g/Clindamycin 600 mg, Azithromycin/Clarithromycin 500 mg, 30–60 minutes pre-operative |
| 1. Prosthetic cardiac valves, including transcatheter-implanted prostheses and homografts | ||||
| 2. Prosthetic material used for cardiac valve repair, such | ||||
| as annuloplasty rings and chords | ||||
| 3. Previous IE | ||||
| 4. Unrepaired cyanotic congenital heart disease or repaired congenital heart disease, with residual shunts or valvular regurgitation at the site of or adjacent to the site of a prosthetic patch or prosthetic device | ||||
| 5. Cardiac transplant with valve regurgitation due to a structurally abnormal valve |
A more detailed review of the NICE and the published guidelines is essential to understand how this very different interpretation of the same data could have arisen. The NICE—although officially a non-departmental public body (NDPB)—is scientifically less independent than the AHA and the ESC, being subject to considerable political influence by the Department of Health and Social Care of the British government (4). In contrast to the AHA and ESC guidelines, where review panels consist of 30–50 independent clinicians, including cardiologist, dentist and infectious disease experts and final recommendations are usually adopted by consensus, the NICE guidelines are developed primarily by a standing committee, that works on multiple guidelines and topic-specific, consulting experts as well as patient representatives and care providers.
The cessation of antibiotic prophylaxis prior to dental procedures as advice in the NICE guidelines is mainly explained by a lack of robust evidence from the available scientific data. In fact, almost no data from randomized clinical trials (RCT) on the effectiveness of antibiotic prophylaxis in the context of dental procedures to prevent infective endocarditis are available, most likely because the conduct of such studies is ethically highly controversial. Excluding conclusive animal studies from the outset as well as data from case control studies and any kind of observational studies from the literature review has led to the alleged lack of evidence promoted in the NICE guidelines.
Besides the criticism of the supposed lack of robust evidence for antibiotic administration, cost-effectiveness seems to play a predominant role in the NICE guideline and the resulting recommendations. The primary emphasis on the cost to the health care system of prescribing antibiotics as a prophylaxis raises legitimate questions about a possible political motivation of the guidelines.
Although the single-handed approach of the British NICE to advice against any prophylactic, antibiotic therapy is difficult to understand and highly questionable given the unity of the leading North American and European societies, a unique situation has arisen. For the first time, European data on the direct impact of the abandonment of antibiotic prophylaxis prior to dental interventions in patients at risk of endocarditis in a large nationwide cohort is available, which paradoxically means that the NICE guideline itself has contributed to new evidence, regarding the necessity of antibiotic prophylaxis.
Considering this data, in 2015 Dayer et al. published an observational large-scale study regarding the incidence of infective endocarditis between 2000 and 2013 in England (55). The authors, among them leading British and U.S. dentists and cardiologist, reported a significant increase in the incidence of patients with infective endocarditis and a significant decreased prescription of antibiotics over the same period of time. It can be stated that one of the objectives of the NICE, namely to structure costs for the health care system more effectively by reducing the prescription of antibiotics, has apparently been achieved over the observed period. As this data is derived from an observational study once more, the grade of evidence has to be discussed as well as conclusions about the causality of cessation of antibiotic prescription and increased incidence of IE. Two other surveys conducted in the U.S. did not observe an increased incidence of IE after the adaptation of the 1997 AHA guidelines in 2007 and the associated abandonment of antibiotic prophylaxis in patients at moderate risk, indicating the clinical effectiveness of the more rigorous recommendation (56,57).
Despite the published data, the updated NICE guidelines released in 2015 (58) maintained to its original advise against antibiotic prophylaxis as allegedly no further evidence on a sufficient level has been published, obviously grading the level of evidence of the study by Dayer et al. as not sufficient. The updated guidelines of the ESC in 2015 (3) and the AHA in 2017 (42) on the other hand evaluated the available literature differently, reaffirming their recommendation for antibiotic prophylaxis prior to invasive dental procedures in patients at increased risk for IE and at high risk for adverse outcomes from IE. In its guideline published in 2016, the British Dental Association (BDA) reacted on the NICE guidelines directly, discussing the risk-benefit profile of antibiotic prophylaxis extensively comparing potential adverse drug reaction (ADR) related with antibiotic prophylaxis and cases of IE that could result in cessation of prophylaxis (4). They concluded that the risk of triggering a disease with high morbidity or mortality through dental therapy if antibiotic prophylaxis is completely abandoned is significantly higher than the risk of ADR related with the frequency of dental measures and the resulting frequency of antibiotic prevention with a rigid recommendation. In fact, considering the data regarding ADRs (59,60) and data published by Dayer et al. (55) cessation of antibiotic prophylaxis as recommended by NICE potentially results in over 400 extra cases of IE including more than 60 possibly fatal courses. On the other hand, according to Thornhill et al. (4) application of antibiotic prophylaxis as recommended by the ESC (3) could lead to seven ADRs annually including one death in three years. Following Cahill et al. (9) the restriction of antibiotic prophylaxis to amoxicillin or an alternative to clindamycin could even decrease ADR to two cases per year without any fatal courses. These projections impressively suggest the rationale of the currently valid European and North American guidelines and make the NICE guidelines even more difficult to follow.
Since a change in this confusing and contradictory recommendation situation is not foreseeable, clinicians faced with the decision to prescribe antibiotic prophylaxis in patients with an increased risk for IE should not only consider national guidelines, but also international recommendations. To ensure responsible and well-balanced therapy practitioners should more over regularly observe the scientific discourse and review latest updates. Eventually, it has to be emphasized that besides antibiotic prophylaxis prior to certain dental procedures the maintenance of good oral health in patients at risk seems to play an even more important role in preventing IE (1,12,41,42,61,62), since persisting odontogenic infections may remain undetected (61) causing bacteremia with high frequency and intensity during every-day activities (38). Therefore, the prevention of oro-dental infections and the consistent adherence to oral maintenance care including regular dental and periodontal examinations as well as prophylaxis are crucial in the prevention of infective endocarditis, especially in patients at increased risk for IE and those at high risk of severe disease outcome (4,9,12).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yskert von Kodolitsch, Harald Kaemmerer, Koichiro Niwa) for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part IV” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-908
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-908). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part IV” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007;116:1736-54. [Crossref] [PubMed]
- Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015;132:1435-86. [Crossref] [PubMed]
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. [Crossref] [PubMed]
- Thornhill MH, Dayer M, Lockhart PB, et al. Guidelines on prophylaxis to prevent infective endocarditis. Br Dent J 2016;220:51-6. [Crossref] [PubMed]
- Holland TL, Baddour LM, Bayer AS, et al. Infective endocarditis. Nat Rev Dis Primers 2016;2:16059. [Crossref] [PubMed]
- Mylotte D, Pilote L, Ionescu-Ittu R, et al. Specialized adult congenital heart disease care: the impact of policy on mortality. Circulation 2014;129:1804-12. [Crossref] [PubMed]
- Hays LH Infective endocarditis: call for education of adults with CHD: review of the evidence. Cardiol Young 2016;26:426-30. [Crossref] [PubMed]
- Moreillon P, Overholser CD, Malinverni R, et al. Predictors of endocarditis in isolates from cultures of blood following dental extractions in rats with periodontal disease. J Infect Dis 1988;157:990-5. [Crossref] [PubMed]
- Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2016;387:882-93. [Crossref] [PubMed]
- Selton-Suty C, Célard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 2012;54:1230-9. [Crossref] [PubMed]
- Palmer RJ. Composition and development of oral bacterial communities. Periodontol 2000 2014;64:20-39. [Crossref] [PubMed]
- Lockhart PB, Brennan MT, Thornhill M, et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc 2009;140:1238-44. [Crossref] [PubMed]
- Viscount HB, Munro CL, Burnette-Curley D, et al. Immunization with FimA protects against Streptococcus parasanguis endocarditis in rats. Infect Immun 1997;65:994-1002. [Crossref] [PubMed]
- Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 2009;30:2369-413. [Crossref] [PubMed]
- Que YA, Haefliger JA, Piroth L, et al. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J Exp Med 2005;201:1627-35. [Crossref] [PubMed]
- Moreillon P, Que YA, Bayer AS. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect Dis Clin North Am 2002;16:297-318. [Crossref] [PubMed]
- Pitts NB, Zero DT, Marsh PD, et al. Dental caries. Nat Rev Dis Primers 2017;3:17030. [Crossref] [PubMed]
- Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 1994;8:263-71. [Crossref] [PubMed]
- Takahashi N, Nyvad B. Caries ecology revisited: microbial dynamics and the caries process. Caries Res 2008;42:409-18. [Crossref] [PubMed]
- Bowen WH, Burne RA, Wu H, et al. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol 2018;26:229-42. [Crossref] [PubMed]
- Simón-Soro A, Mira A. Solving the etiology of dental caries. Trends Microbiol 2015;23:76-82. [Crossref] [PubMed]
- Arif N, Sheehy EC, Do T, et al. Diversity of Veillonella spp. from sound and carious sites in children. J Dent Res 2008;87:278-82. [Crossref] [PubMed]
- Kleinberg I. A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med 2002;13:108-25. [Crossref] [PubMed]
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369:51-9. [Crossref] [PubMed]
- Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc 2000;131:887-99. [Crossref] [PubMed]
- Featherstone JD. The continuum of dental caries--evidence for a dynamic disease process. J Dent Res 2004;83 spec no C:C39-42.
- Pitts NB. Modern concepts of caries measurement. J Dent Res 2004;83 spec no C:C43-7.
- Siqueira JF, Antunes HS, Rôças IN, et al. Microbiome in the Apical Root Canal System of Teeth with Post-Treatment Apical Periodontitis. PLoS One 2016;11:e0162887. [Crossref] [PubMed]
- Siqueira JF, Rôças IN. Exploiting molecular methods to explore endodontic infections: Part 2--Redefining the endodontic microbiota. J Endod 2005;31:488-98. [Crossref] [PubMed]
- Pinheiro ET, Gomes BP, Ferraz CC, et al. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J 2003;36:1-11. [Crossref] [PubMed]
- Sanz M, Herrera D, Kebschull M, et al. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J Clin Periodontol 2020;47:4-60. [Crossref] [PubMed]
- Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 2014;35:3-11. [Crossref] [PubMed]
- Wade WG. Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease? J Clin Periodontol 2011;38:7-16. [Crossref] [PubMed]
- Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J 2012;6:1176-85. [Crossref] [PubMed]
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol 1998;25:134-44. [Crossref] [PubMed]
- Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1997;277:1794-801. [Crossref] [PubMed]
- Cotti E, Mercuro G. Apical periodontitis and cardiovascular diseases: previous findings and ongoing research. Int Endod J 2015;48:926-32. [Crossref] [PubMed]
- Lockhart PB, Brennan MT, Sasser HC, et al. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008;117:3118-25. [Crossref] [PubMed]
- Pallasch TJ, Slots J. Antibiotic prophylaxis and the medically compromised patient. Periodontol 2000 1996;10:107-38. [Crossref] [PubMed]
- Bender IB, Naidorf IJ, Garvey GJ. Bacterial endocarditis: a consideration for physician and dentist. J Am Dent Assoc 1984;109:415-20. [Crossref] [PubMed]
- Duval X, Leport C. Prophylaxis of infective endocarditis: current tendencies, continuing controversies. Lancet Infect Dis 2008;8:225-32. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
- Jones T, Baumgartner L, Bellows MT, et al. Committee on Prevention of Rheumatic Fever and Bacterial Endocarditis, American Heart Association. Prevention of rheumatic fever and bacterial endocarditis through control of streptococcal infections. Circulation 1955;11:317-20.
- Rammelkamp C. Committee on Prevention of Rheumatic Fever and Bacterial Endocarditis, American Heart Association. Prevention of rheumatic fever and bacterial endocarditis through control of streptococcal infections. Circulation 1957;15:154-8. [Crossref]
- American Heart Association. Prevention of Rheumatic Fever and Bacterial Endocarditis Through Control of Streptococcal Infections. Circulation 1960;21:151-5. [Crossref]
- Wannamaker LW, Denny FW, Diehl A, et al. Prevention of Bacterial Endocarditis. Circulation 1965;31:953-4. [Crossref] [PubMed]
- Rheumatic Fever Committee and the Committee on Congenital Cardiac Defects. A.H.A., Prevention of bacterial endocarditis. Circulation 1972;46:S3-S6.
- Kaplan EL. Prevention of bacterial endocarditis. Circulation 1977;56:139A-43A. [PubMed]
- Shulman ST, Amren DP, Bisno AL, et al. Prevention of Bacterial Endocarditis. A statement for health professionals by the Committee on Rheumatic Fever and Infective Endocarditis of the Council on Cardiovascular Disease in the Young. Circulation 1984;70:1123A-7A. [PubMed]
- Dajani AS, Bisno AL, Chung KJ, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1990;264:2919-22. [Crossref] [PubMed]
- Horstkotte D, Follath F, Gutschik E, et al. Guidelines on prevention, diagnosis and treatment of infective endocarditis executive summary; the task force on infective endocarditis of the European society of cardiology. Eur Heart J 2004;25:267-76. [Crossref] [PubMed]
- Neidenbach RC, Lummert E, Vigl M, et al. Non-cardiac comorbidities in adults with inherited and congenital heart disease: report from a single center experience of more than 800 consecutive patients. Cardiovasc Diagn Ther 2018;8:423-31. [Crossref] [PubMed]
- Albes JM. Current practice in prophylaxis of endocarditis: are we running into trouble? Cardiovasc Diagn Ther 2018;8:423-31.
- (NICE), N.I.f.H.a.C.E., Prophylaxis Against Infective Endocarditis: Antimicrobial Prophylaxis Against Infective Endocarditis in Adults and Children Undergoing Interventional Procedures. 2008, National Institute for Health and Clinical Excellence (UK) Copyright © 2008, National Institute for Health and Clinical Excellence.
- Dayer MJ, Jones S, Prendergast B, et al. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet 2015;385:1219-28. [Crossref] [PubMed]
- Desimone DC, Tleyjeh IM, Correa de Sa DD, et al. Incidence of Infective Endocarditis Caused by Viridans Group Streptococci Before and After Publication of the 2007 American Heart Association's Endocarditis Prevention Guidelines. Circulation 2012;126:60-4. [Crossref] [PubMed]
- Pasquali SK, He X, Mohamad Z, et al. Trends in endocarditis hospitalizations at US children's hospitals: impact of the 2007 American Heart Association Antibiotic Prophylaxis Guidelines. Am Heart J 2012;163:894-9. [Crossref] [PubMed]
- National Institute für Health and Care Excellence (NICE). Prophylaxis Against Infective Endocarditis: Antimicrobial Prophylaxis Against Infective Endocarditis in Adults and Children Undergoing Interventional Procedures. 2015: p. Available online: https://www.nice.org.uk/guidance/cg64. (accessed September 2020).
- Thornhill MH, Dayer MJ, Prendergast B, et al. Incidence and nature of adverse reactions to antibiotics used as endocarditis prophylaxis. J Antimicrob Chemother 2015;70:2382-8. [Crossref] [PubMed]
- Lee P, Shanson D. Results of a UK survey of fatal anaphylaxis after oral amoxicillin. J Antimicrob Chemother 2007;60:1172-3. [Crossref] [PubMed]
- Folwaczny M, Bauer F, Grünberg C. Significance of oral health in adult patients with congenital heart disease. Cardiovasc Diagn Ther 2019;9:S377-87. [Crossref] [PubMed]
- Folwaczny M, Wilberg S, Bumm C, et al. Oral Health in Adults with Congenital Heart Disease. J Clin Med 2019;8:1255. [Crossref] [PubMed]


