Evading the fate of Pheidippides: acute coronary thrombosis in a young marathon runner with minimal atherosclerosis but sickle cell trait
Introduction
After running across the plain of Marathon in 430 BC, Some myths suggest that Pheidippides delivered his message of victory to the Athenians and then died suddenly. It is known today that marathon running transiently increases the risk of sudden cardiac death (1). Although there are many mechanisms that could precipitate such a catastrophe; we present a case with intra-vascular evidence that occlusive coronary thrombosis can occur from only minimal atherosclerosis.
Case report
A previously fit and well 42-year-old male was admitted to our tertiary cardiac centre experiencing chest pain compatible with acute myocardial infarction. Earlier that day, he had competed in a local marathon and noticed some mild chest discomfort at around eight miles. Due to his physical fitness he was able to continue on and complete the marathon. During his post-marathon ablutions, he felt some further symptoms but was able to complete eating a meal before the onset of severe pain radiating to the back, neck, jaw and arm developed. At this point he realised that a “heart attack” was a possibility and his wife called an ambulance.
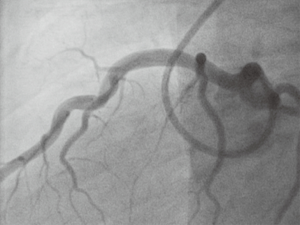
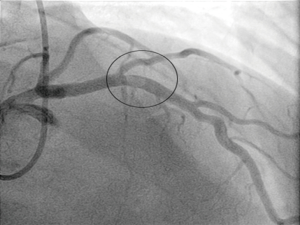
Subsequent emergency coronary angiography revealed a large thrombus in the proximal left anterior descending (LAD) coronary artery (Figures 1,2). The position of this clot is generally accepted as one of the most high-risk areas within the coronary tree for developing a fatal event, due to the large area of myocardium subtended by this vessel. Despite attempts with a thrombus aspiration catheter, the thrombus was too large to retrieve with conventional tools. A decision was made to employ medical anti-platelet treatment and full anti-thrombotic therapy (LMWH) in the first instance. He was re-studied by angiography again at day 4 (Figures 3,4). Due to the fact that the patient was very fit before this event, his proximal LAD artery was normal and a very large vessel. Direct imaging was performed with intravascular ultrasound and virtual histology. This is a small ultrasound probe able to enter the coronary artery and give direct images of any pathology in-situ, live in the catheter lab (Figures 5,6).



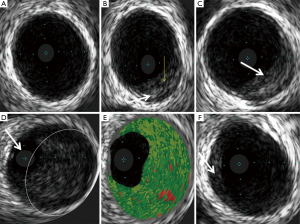
This patient’s artery measured 6 mm in diameter on intravascular ultrasound (normal fit adult male reference 3-4 mm). This fact alone appeared to allow reasonable flow to continue within the artery due to preserved luminal patency. This prevented the patient from blocking the artery completely with thrombus and avoided any significant infarction.
The intravascular ultrasound was very instructive regarding the nature of this event. Figures 5B,6 shows the small rim of plaque (white arrow) with evidence of superficial erosion/fissuring (yellow arrow). Figure 5C is taken a few millimetres further down the vessel and shows early organisation of thrombotic material (white arrow) across the fissured plaque. Figure 5D clearly shows the main thrombotic occlusion (within white circle) in the vessel and the residual lumen filled with the ultrasound probe (white arrow). Figure 5E is the virtual histology (colour-coded tissue characterisation) generated from the backscatter of ultrasound signals, indicating that the composition is similar to that generated by thrombus (5). Figure 5F shows the internal anatomy of the vessel distal to the thrombus, this again shows very minimal plaque disease (white arrow) consistent with early atherosclerosis.
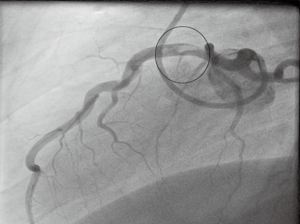
Reasonably good blood flow continued to the myocardium, despite the significant occlusion. Over a period of days the patient improved and after two weeks full treatment (one week in hospital and one week out) a further angiogram (Figures 7,8) was performed showing complete resolution of the thrombus with no obvious residual angiographic stenosis or disease. A subsequent bubble-echocardiogram confirmed normal left ventricular function, with no evidence of myocardial infarction. A patent foramen ovale/atrial septal defect was also excluded with this study, ruling out paradoxical embolism as a cause. We did however find that the patient had sickle cell trait on subsequent blood tests. This provided an alternative hypothesis for the enhanced thrombotic response following extreme exertion and physiological stress.

Discussion
A dichotomous conundrum appears to exist between the clear beneficial effects of regular physical exertion and the increased risk generated by some forms of exercise (7). With regard to marathon participation, the actual risk of death has been quoted around 1 per 80,000 participants from a report of events at the London Marathon over 25 years (8). Epidemiological studies have proven that at a mean age of 46, 75% of sudden cardiac deaths during marathon running were due to confirmed coronary artery disease (9). Plaque rupture with thrombosis is, on autopsy, the most common mechanism, similar to what we have witnessed, in vivo, in our case.
Extreme physical exertion places stress on the body and can trigger: muscle rhabdomyolysis; inflammation; cytokine release; platelet activation; alterations in sympathetic and parasympathetic tone; coronary vasoconstriction and lactic acidosis. Moreover, there are the effects of increasing blood pressure, heart rate and shear stress on coronary plaques (9). In 1987, Kark et al. (10) published in the New England Journal of Medicine data gathered from two million army recruits. This appeared to show that those in basic military training were at a greater risk of exercise-related sudden death if they had sickle cell trait and this was from an “unknown mechanism”.
In our case, it is proposed that initial plaque fissure occurred around eight miles into the marathon but due to the heart rate and blood pressure generated by the physical exertion, conditions were not right for a thrombus to propagate at that stage. In the recovery phase when both heart rate and blood pressure fell, flow is likely to have become more sluggish at the site of injury. The additive effects of dehydration, sickle cell trait and a heightened coagulation system may therefore have propagated this large coronary thrombus. The interesting irony is that had the patient not been so highly trained in the first place, with such large coronary arteries, he may have occluded the artery earlier and potentially died suddenly.
Conclusions
These findings should alert those involved in Marathons, either as a participant or as a coach, that even the presence of minimal coronary artery disease can be enough (with the right conditions) to precipitate coronary thrombotic events. The knowledge that sickle cell trait could add risk in extremes of exertion should also be highlighted. This may lead to the consideration of more screening for concurrent coronary artery disease and other coagulation disorders (11) and more discussion about pharmacological interventions, such as anti-platelet treatment, before marathons (12). This approach would need to be reserved for “high risk” participants as there is not enough current evidence to say that it could prevent subsequent events.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003;107:3109-16. [PubMed]
- Murray SW, Cooper RM, Mills JD, et al. Lateral angiographic cine projection showing clear evidence of a large thrombus in the proximal LAD. Asvide 2015;2:075. Available online: http://www.asvide.com/articles/622
- Murray SW, Cooper RM, Mills JD, et al. Lateral angiographic cine projection of Figure 3. Asvide 2015;2:076. Available online: http://www.asvide.com/articles/623
- Murray SW, Cooper RM, Mills JD, et al. Real-time Intravascular ultrasound pullback through the culprit disease. Asvide 2015;2:077. Available online: http://www.asvide.com/articles/624
- Murray SW, Palmer ND. Intravascular ultrasound and virtual histology interpretation of plaque rupture and thrombus in acute coronary syndromes. Heart 2009;95:1494. [PubMed]
- Murray SW, Cooper RM, Mills JD, et al. Final lateral angiogram showing complete resolution of thrombosis and TIMI III flow. Asvide 2015;2:078. Available online: http://www.asvide.com/articles/625
- Mittleman MA, Maclure M, Tofler GH, et al. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med 1993;329:1677-83. [PubMed]
- Tunstall Pedoe DS. Marathon cardiac deaths: the london experience. Sports Med 2007;37:448-50. [PubMed]
- O'Keefe JH, Patil HR, Lavie CJ, et al. Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin Proc 2012;87:587-95. [PubMed]
- Kark JA, Posey DM, Schumacher HR, et al. Sickle-cell trait as a risk factor for sudden death in physical training. N Engl J Med 1987;317:781-7. [PubMed]
- Möhlenkamp S, Lehmann N, Breuckmann F, et al. Running: the risk of coronary events: Prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J 2008;29:1903-10. [PubMed]
- Siegel AJ. Pheidippides redux: reducing risk for acute cardiac events during marathon running. Am J Med 2012;125:630-5. [PubMed]