Preclinical evaluation of a novel abluminal surface coated sirolimus eluting stent with biodegradable polymer matrix
Introduction
Percutaneous coronary intervention (PCI), a less invasive procedure than coronary artery bypass grafting (CABG), has been widely used for the treatment of coronary artery obstruction. The existing interventional medical devices are used in these procedures are associated with restenosis. To deal with restenosis drug eluting stents (DES) were introduced.
Introduction of DES primarily proven in-stent neointimal formation reduction and thereby minimized the occurrence of restenosis compare to bare-metal stents (BMS).
Although first-generation DES, Cypher (Cordis Corporation, J&J, NJ, USA) and Taxus (Boston Scientific Corporation, Natick, Mass, USA) were successfully achieved restenosis rate reduction. However, safety related issues of first generation DES; inflammatory response, delayed healing causing late and very late thrombosis, and local drug toxicity were unanswered (1-9).
Both Cypher and Taxus were designed using durable polymers to carry and control the release of their antiproliferative agents. The permanent presence of these polymers has been correlated to local inflammatory responses and local toxicity in various preclinical analysis (10-13). Furthermore, durable polymers used in first-generation DES were associated with mechanical complications like polymer delamination and webbing which may create stent expansion issues (14) and non-uniform coating resulting in erratic drug distribution. Therefore safety issue remains unresolved in first generation DES. Second generation DES were focused on novel drug carrier systems including absorbable (or biodegradable) polymers and modified polymers (14-17).
Additional improvements includes, development of more modern platforms (e.g. , better deliverability, radio-opacity, flexibility, and radial strength) as well as the use of novel antiproliferative agents or reduced doses of current approved antiproliferative drugs.
Current approaches deliver the drug through entire stent surface i.e. , inside and outside wall of the stent. Hypothetically any cytostatic or cytotoxic drug inhibits ECM building inside wall of the stent. Therefore DES with bio-degradable polymers and limited drug delivery through abluminal surface of the struts is gaining more and more importance (18-20).
The present in vitro and in vivo study has been designed to investigate the preclinical safety and efficacy of the Abluminus™ stent (SES). The stent contains sirolimus drug with mixture of biodegradable polymers coated on abluminal surface of stent and exposed surface of the balloon.
Materials and methods
PLLA, PLGA (50:50 lactide-co-glycolide) was obtained from Lakeshore Biomaterials and the rapamycin (sirolimus) was purchased from Biocon Ltd. , India. The coated stents were prepared by spray-coating method as per reported method (21). In brief, 1% drug solution with polymers in dichloromethane was sprayed on the targeted stent surface and was allowed to dry for 10 minutes. Bare metal stent (BMS) was made of L605 alloy, were 16-20 mm in length and struts thickness of 73 µm which was also used as base platform of sirolimus eluting stent and polymer only coated stent.
Drug release profile of the stent samples were analysed by soaking in Hank’s solution. The samples were incubated at 37 °C with gentle rotation at 70 RPM. The elution volume was 2 mL and the entire volume was sampled and replenished at each time point. The elution curves were generated by measuring the amount of drug each stent, released into Hank’s solution by UV at 278 nm after 0, 1, 7, 14, 30 and 60 days.
Device description
Abluminus™/Mitigator™/Nostrum™ (Envision Scientific Pvt. Ltd. , India) is a pre-crimped stent coated with sirolimus on abluminal surface of the stent and balloon catheter. The novelty of the product was an abluminal coating of drug polymer matrix on the stent surface and exposed balloon surface in pre-crimped configuration. The schematic coating is shown in Figure 1.

Study design
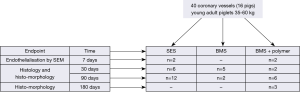
The present study was performed as shown in Figure 2. In present investigation SES was used as test device, while two controls were BMS and polymer only coating on abluminal surface (PC).
The study was performed with the objective of demonstrating the effect of the SES on healing and neointimal proliferation by using histology and histomorphometry. A total of 16 animals (n=40) were included in this study. The animals were divided into four groups, according to the follow-up time (7, 30, 90 or 180 days). The two different control devices were compared with the SES stents. Endothelialization was evaluated at 7 days on SES and PC. For histology and histomorphometry, at 30-days, 13 stent were explanted (n=6 for SES, n=5 for BMS and n=2 for PC) and at 90 days 20 stents were explanted (n=12 for SES, n=2 for BMS and n=6 for PC). PC (n=3) stents were explanted at 180 days for polymer degradation evaluation.
The coronary angiographies were carried out after each implant and at each follow-up. The angiograms confirmed patency of all the treated vessels. Thrombus presence, incomplete stent strut apposition and myocardial infarctions were not detected in any of implanted groups either by morphological heart examination or by histology.
Investigations was followed the CPCSEA (Committee for the purpose of control and supervision of experiments on animals) Guidelines 1998 and the Guide for the Care and Use of Laboratory Animals, Indian National Science Academy, India, and those of the Italian Ministry of Health, Italy. The study protocol was approved by local ethical committee.
Safety and efficacy porcine study
Stent procedure description
Domestic young adult piglets (35 to 60 kg) were anesthetized and treated with antibiotics, then heparinised 150 (IU/kg) by intra-arterial route (ACT >300 sec. ). Surgical access was obtained through common carotid artery using 7 Fr introducer. A Judkins or Amplaz type guide catheter was introduced through the introducer by means of a 0.035 inch. The appropriate stent was delivered to the intended site over a guide wire with the use of fluoroscopic guidance, and stent deployment was performed using a 1:1.1 stent-to-artery diameter ratio. The devices were then expanded using appropriate pressures until they were well apposed to the vessel wall. Animals were recovered and housed until their designated day of euthanasia.
The antiplatelet treatment was limited to Aspirin® from two days before to the 30 day after the intervention and then suspended. No supplementary antiplatelet drugs (ticlopidine or clopidogrel) were administered.
At the end of the scheduled follow-up, each group of animals underwent a further angiographic examination in order to assess the vessel patency. The animals were anesthetised with intramuscular injections of 2 mg/kg di-ketamine and 2 mg/kg of xylazine and by inhaling iso-fluoran 1%. A 7 Fr introducer was positioned in the left carotid artery and a follow-up angiography was carried out on the implanted coronaries using a similar method to that described above. The animals received 14,000 IU of heparin intravenously administered. The animals were then euthanised by an intravenous injection of barbiturate (60 mg/kg) through the jugular access following the “AVMA guidelines on euthanasia (June 2007)”.
After the explant, the heart was submitted to X-ray analysis to confirm stent location. Hearts were excised and pressure perfused with a heparinised physiological solution until cleared of haematic traces. The stented arterial segments were then carefully dissected free from the heart.
The coronaries were fixed with a solution of formaldehyde at 4% at 150 mm/Hg pressure. Finally the coronary vessels were carefully dissected from epicardial surface preserving artery size and shape.
Histology protocol
Formalin post-fixed stent samples were analyzed for endothelialization by scanning electron microscopy (SEM). Stented segments for SEM were cut in longitudinally sections and were dried. Stereo microscope pictures were taken at high magnification before gold sputtering. At 30, 90 and 180 day follow-up coronary arteries were dissected and stented coronary artery segments were processed for methyl-methacrylate embedding, while proximal and distal unstented vessel sections were paraffin embedded.
A representative number of 4 µm sections were cut perpendicularly to the long axis of the vessel by a precision microtome, at the proximal, mid and distal stent level and at proximal and distal unstented tracts. Histological sections were stained with haematoxylin and eosin (H&E) and Movat’s pentachromic stains to evidence elastic fibers; photomicrographs were taken for each segment of interest.
A vessel injury score was calculated according to the Schwartz method (22). Cross-sectional areas (external elastic lamina, internal elastic lamina, and lumen) of each section were measured with morphometry. Neointimal thickness was measured as the distance from the inner surface of each stent strut to the luminal border. Data were collected on each stent section and included strut apposition to the vessel wall, fibrin deposition around struts, granulomas, haemorrhage and giant cells around stent struts and were expressed as a percentage of the total number of struts in each section.
Histomorphometry
Endothelialization was calculated from the stereoscopic images measuring the non-coated strut surfaces over the total extent of strut surfaces. Neointimal thickness and neointimal area were measured on histological images, and percentage stenosis was consequently calculated. Patency was evaluated as percent of in-stent area stenosis, endothelialization percent and histomorphometric variables were analysed by Image-Pro Plus® software (Media Cybernetics Inc. , Bethesda, MD, USA).
Polymer absorption study
Animals in PC group were additionally analysed for histomorphometric and histo-pathological measurements of residual polymer at 30, 90 and 180 days. Polymer thickness was measured at all struts included transverse section cut at defined time points. Minimum, maximum and mean values were reported for each evaluated stent.
Statistical analysis
The data are presented as mean ± SD for parametric data and frequency percentage for scores. Comparison among continuous variables were performed using one way ANOVA as data was normally distributed, adopting the Bonferroni t-test method for multiple comparison. Differences among inflammation scores were analysed with the Kruskal-Wallis test. Statistical analysis was performed using SAS 9.1.3 software (SAS Institute Inc. , Cary, NC, USA). A P value of 0.05 or less was considered statistically significant.
Results
In vitro drug release
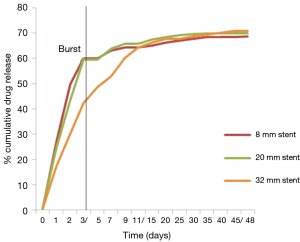
Small, medium and long size stent (n=9) were tested for in-vitro drug release. The drug showed biphasic drug release; initially first 3-4 days burst release and then controlled release up to 48 days. Burst release was 40-50% in 3-4 days and rest 50% drug was released in 40-42 days (Figure 3). First order release kinetics was observed and drug release kinetics was similar in all length sizes.
In vivo drug loss
Whole blood was collected from the animals at each time interval (7, 30 and 90 days) for evaluation of drug content. The results did not show presence of sirolimus drug systemically.
Implant distribution
A total of 16 juvenile domestic pigs randomly received two or three stent implant in orthotopic position (at least one stent in the left and one stent in the right coronary artery) using 40 investigational stents. The average implant pressure was 10.8 atm. Two pigs (n=2 for SES and n=2 for PC) were sacrificed at seven days to conduct the SEM investigations and remaining animals were sacrificed at longer term follow-up. Thirteen stents were explanted at 30 days and 20 stents were explanted at 90 days follow-up for histological and histomorphometric examinations. The coronary angiographies carried out after implant and at each follow-up confirmed the patency of all treated vessels. Three stents were dissected at 180 days for histomorphometry.
Endothelialization at seven days
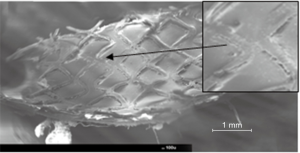
The specimens obtained from the stent longitudinal sections were observed by SEM in order to evaluate and measure the vessel healing in terms of quality and the percent extent of neo-endothelialization.
In Figure 4, the appearance at SEM examination of SES at seven days of implant is represented. Specimen surfaces SES, appears free from platelet aggregation and thrombotic clots. Morphometric assessment of the surface endothelialization was quantified in percent of strut coverage by a continuous carpet of endothelial cells. The results of these measurements indicate that endothelial cells covered uniformly almost 100% of the struts surface.
Histology evaluation
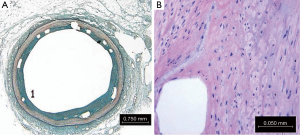
Histological evaluation for defined attributes was performed in all the three groups: SES, BMS and PC. The 30-day tissue response was in general minimal, with negligible infiltration of inflammatory cells (Figure 5). The neointimal surface was lined by a regular carpet of endothelium. Smooth muscle cells around the stent strut were also observed.
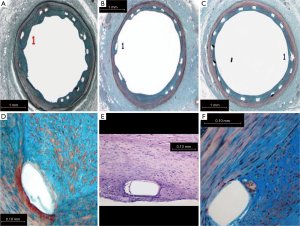
Neointimal growth was non-obstructive, characterised by an even distribution of a moderate number of cells in a proteoglycan matrix. Blood clots and minor fibrin deposits, mainly localised at strut proximity, were seen. The values were recorded at both the time points: 30 and 90 days. Cell populations [monocytes, polymorphonucleates (PMN) with smooth muscle cells the most numerous] and their density were assessed in proximal, mid and distal stent sections and showed no statistical differences between the two groups (Table 1).
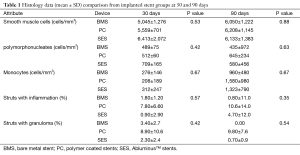
Full table
The inflammation score was mild in general in all the stent groups, without statistical differences. Comparatively inflammation was higher in the PC stent group; also struts with granulomas were seen more remarkably in this group. SES and BMS are less stimulated the infiltration of monocytes, with a very low density, while, on the contrary their density was higher in the PC stent group at 30 and 90 days.
At 90 days neointimal thickness maintained lower for the SES group (0.18±0.10 mm) as compared to the BMS (0.61±0.09 mm) and PC group (0.59±0.08 mm), (P<0.001). Neointimal area was also lower in the SES group as compared to the BMS and PC group (DES 1.1±0.55 mm2; BMS 1.92±0.36 mm2; PC 1.94±0.48 mm2; P<0.05). The percentage area stenosis was also maintained significantly lower in SES compared to the BMS and PC group (SES 18%±11% vs. BMS 30%±12% vs. BMS + polymer only 32%±14%, P<0.05). Also the medial area was higher in the BMS and PC group as compared to the SES group and were not statistically significant (BMS 2.82±0.81 mm2, PC group 2.98±0.69 mm2, SES 2.38±0.78 mm2; P= ns) (Figure 6).
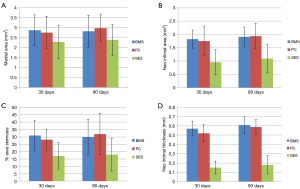
Polymer absorption and thickness evaluation
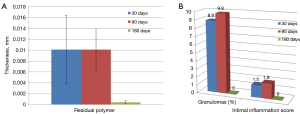
At 180 days minimal and maximal polymer thickness, as well as mean polymer thickness were reduced as compared to 30 and 90 days.
Figure 7 shows that tissue response to polymer absorption in PC group at 180 days. No inflammatory response was observed at this time point. At 180 days residual minimal, maximal and mean polymer thickness was remarkably reduced (nearly complete absorbed) as shown in Figure 8A. Also inflammation response was absent at 180 days in PC group (Figure 8B). High magnification imaging was used to study and quantify in PC group.
Discussion
Endothelialization is critical step of vascular healing due to injury in vessel due to stent implantation. The origin of new endothelium may be from either the adjacent recruitment of endothelial cells (23). Circulating endothelial progenitor cells (EPC) have been shown to participate in repair of damaged or degenerating vascular surfaces (24,25).
Due to cytostatic or cytotoxic properties of certain drugs, a non-selective inhibition of all cells occurs in stent healing process (26-30). Non-selective inhibition results in alteration of vascular healing. Maintain sustained control of smooth cell proliferation in arterial wall without effecting extracellular matrix building process on luminal side is yet major challenge in present DESs (31-34). Selective drug elution from the stent surface is important and can be explained by abluminal coating on the stent and exposed surface of the balloon with control drug elution. Present sirolimus elution through abluminal surface of the stent and exposed surface of the balloon can reduces focal restenosis by more homogeneous drug delivery in artery and also take care of neointimal proliferation without hindrance of ECM building process on the luminal struts. This can demonstrate safety concerns related to the problems associated with first generation DES.
The study was mainly focused on safety and efficacy of abluminal coating sirolimus eluting stent formulated with absorbable polymers matrix. The SES showed early time 100% endothelialization at 7 day compare to historical non-polymeric DES (Cypher & Taxus) data (34).
Thrombus, incomplete stent strut apposition and myocardial infarctions were not detected in any of implanted groups either by morphological heart examination or by histology. Injury scores were mild in all groups with decreasing pattern from 30 to 90 days. Fibrin was mildly present in few sections at 30 days in all three groups and was disappeared at 90 days.
Inflammation was minimal at 30 days in all three groups and was increased at 90 days in PC group which was absent at 180 days in same group. Mild inflammatory response increased at 90 days may be more likely due to polymer absorption, further bio-degradation of polymers in to their monomer acids like lactic or glycolic acid leads to acidic pH in local tissues. Decreasing inflammatory trend in SES group shows effect of sirolimus over absorption of polymers. At 180 days, degraded acids might me degraded to carbon dioxide and water. Polymer end group of PLLA or PLGA may have produced less acid moieties and thereby it may have caused less inflammation.
Complete re-sorption of polymer at 180 days have near to null inflammation reaction which indicated marginal lower inflammatory response as compared the higher grade of inflammation in Cypher stent (12). Presence of granulomas compared to Cypher vs. SES vs. PC at 30 days was 14%, 2.30% and 8.9% while at 90 days it was 43%, 0.70% and 9.8% respectively. At 180 days SES showed 0% compare to 60% of Cypher (34). Permanent polymer placements of Cypher stent may have cause larger numbers of granuloma comparatively. No polymer residue at 180 days has resulted in lower granuloma %.
Significant reduction in neointima thickness (NIT) and percentage neointima hyperplasia (% NIH) was observed in SES group. Neointima thickness was 0.15 mm at 30 day follow-up, and remains 0.18 mm at 90 days, while BMS neointima was thicker 0.57 mm at 30 days and was 0.61 mm at 90 days. Similarly, in the PC group, it was 0.52 to 0.59 mm respectively at 30 and 90 days. This difference in neointima thickness between SES and BMS/PC group was statistically significant (P<0.001). This shows that selective drug delivery from stent and balloon able to obstruct neointimal growth without affecting on endothelialization. It was further evidenced by endothelialization at 7 days.
After 30 days, the SES demonstrates intact internal elastic laminae, some mild fibrin deposits and limited haemorrhagic blood clots around few struts. At 180 days, inside the neointima, the healing process appears completed where all fibrin and haemorrhagic traces around the struts disappear without residual inflammation.
Conclusions
Abluminal coated SES, AbluminusTM, NostrumTM and MitigatorTM with biodegradable polymer is safe with no thrombus events, signs of delayed endothelialization and effective in reducing neointimal thickness. The results are yet to establish in human trials.
Acknowledgements
Funding: Study was sponsored by Envision Scientific Private Limited. Sponsor has no role in study design, experiments, analysis, etc. The experiments were performed at Division of Veterinary Pathology, University of Turin.
Footnote
Conflicts of Interest: P Sojitra, A Vyas and B Chevli is employee of Envision Scientific Private Limited. M Doshi is owner of Envision Scientific Private Limited. Other authors declare no conflicts of interest.
References
- Bertrand OF, Sipehia R, Mongrain R, et al. Biocompatibility aspects of new stent technology. J Am Coll Cardiol 1998;32:562-71. [PubMed]
- Nakazawa G, Finn AV, Ladich E, et al. Drug-eluting stent safety: findings from preclinical studies. Expert Rev Cardiovasc Ther 2008;6:1379-91. [PubMed]
- Nakazawa G, Finn AV, Joner M, et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation 2008;118:1138-45. [PubMed]
- Virmani R, Farb A, Guagliumi G, et al. Drug-eluting stents: caution and concerns for long-term outcome. Coron Artery Dis 2004;15:313-8. [PubMed]
- Hezi-Yamit A, Sullivan C, Wong J, et al. Impact of polymer hydrophilicity on biocompatibility: implication for DES polymer design. J Biomed Mater Res A 2009;90:133-41. [PubMed]
- Feres F, Costa JR Jr, Abizaid A. Very late thrombosis after drug-eluting stents. Catheter Cardiovasc Interv 2006;68:83-8. [PubMed]
- Cook S, Wenaweser P, Togni M, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation 2007;115:2426-34. [PubMed]
- Siqueira DA, Abizaid AA, Costa Jde R, et al. Late incomplete apposition after drug-eluting stent implantation: incidence and potential for adverse clinical outcomes. Eur Heart J 2007;28:1304-9. [PubMed]
- Kounis NG, Hahalis G, Theoharides TC. Coronary stents, hypersensitivity reactions, and the Kounis syndrome. J Interv Cardiol 2007;20:314-23. [PubMed]
- Pendyala LK, Li J, Shinke T, et al. Endothelium-dependent vasomotor dysfunction in pig coronary arteries with Paclitaxel-eluting stents is associated with inflammation and oxidative stress. JACC Cardiovasc Interv 2009;2:253-62. [PubMed]
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007;115:1051-8. [PubMed]
- Finn AV, Nakazawa G, Joner M, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol 2007;27:1500-10. [PubMed]
- Silva GV, Fernandes MR, Madonna R, et al. Comparative healing response after sirolimus- and paclitaxel-eluting stent implantation in a pig model of restenosis. Catheter Cardiovasc Interv 2009;73:801-8. [PubMed]
- Smits P. COMPARE Trial. Transcatheter Cardiovascular Therapeutics (TCT). San Francisco, CA, 2009. Available online: http://www.crf.org/tct
- Kumar P, Pillai R, Sreedharan M, et al. RAPSTROM™ first-in-man study long-term results of a biodegradable polymer sustained-release sirolimus-eluting stent in de novo coronary stenoses. J Interv Cardiol 2014;27:373-80. [PubMed]
- Sojitra P, Engineer C, Raval A, et al. Covalently Conjugation of Genistein with Biodegradable Poly L-Lactide. Trends Biomater Artif Organs 2010;23:144-9.
- Granada JF, Inami S, Aboodi MS, et al. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ Cardiovasc Interv 2010;3:257-66. [PubMed]
- Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet 2013;381:651-60. [PubMed]
- Fujimoto Y, Kobayashi Y, Yamaguchi M. . Delamination of abluminal polymer of biolimus-eluting stent. JACC Cardiovasc Interv 2012;5:e5-6. [PubMed]
- Mehilli J, Byrne RA, Wieczorek A, et al. Randomized trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis. Eur Heart J 2008;29:1975-82. [PubMed]
- Doshi M, Sherdiwala D, Sojitra P, et al. Method and system for coating insertable medical devices. US8529983 (2013).
- Schwartz RS, Edelman ER, Carter A, et al. Drug-eluting stents in preclinical studies: recommended evaluation from a consensus group. Circulation 2002;106:1867-73. [PubMed]
- Robinson KA, Roubin G, King S, et al. Correlated microscopic observations of arterial responses to intravascular stenting. Scanning Microsc 1989;3:665-78; discussion 678-9. [PubMed]
- Banerjee S, Brilakis E, Zhang S, et al. Endothelial progenitor cell mobilization after percutaneous coronary intervention. Atherosclerosis 2006;189:70-5. [PubMed]
- Zhao FH, Chen YD, Jin ZN, et al. Are impaired endothelial progenitor cells involved in the processes of late in-stent thrombosis and re-endothelialization of drug-eluting stents? Med Hypotheses 2008;70:512-4. [PubMed]
- Nakazawa G, Finn AV, John MC, et al. The significance of preclinical evaluation of sirolimus-, paclitaxel-, and zotarolimus-eluting stents. Am J Cardiol 2007;100:36M-44M. [PubMed]
- Nakazawa G, Finn AV, Virmani R. Vascular pathology of drug-eluting stents. Herz 2007;32:274-80. [PubMed]
- Barilli A, Visigalli R, Sala R, et al. In human endothelial cells rapamycin causes mTORC2 inhibition and impairs cell viability and function. Cardiovasc Res 2008;78:563-71. [PubMed]
- Butzal M, Loges S, Schweizer M, et al. Rapamycin inhibits proliferation and differentiation of human endothelial progenitor cells in vitro. Exp Cell Res 2004;300:65-71. [PubMed]
- Chen TG, Chen JZ, Wang XX. Effects of rapamycin on number activity and eNOS of endothelial progenitor cells from peripheral blood. Cell Prolif 2006;39:117-25. [PubMed]
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315-23. [PubMed]
- Tsimikas S. . Drug-eluting stents and late adverse clinical outcomes lessons learned, lessons awaited. J Am Coll Cardiol 2006;47:2112-5. [PubMed]
- Waksman R, Buch AN, Torguson R, et al. Long-term clinical outcomes and thrombosis rates of sirolimus-eluting versus paclitaxel-eluting stents in an unselected population with coronary artery disease (REWARDS registry). Am J Cardiol 2007;100:45-51. [PubMed]
- Finn AV, Kolodgie FD, Harnek J, et al. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 2005;112:270-8. [PubMed]