Clinical performance of a novel ultrathin strut, low-dose, sirolimus-eluting stent with abluminal-only biodegradable polymeric coating for patients undergoing percutaneous coronary intervention in the daily practice
Introduction
Since the introduction of the so-called first generation drug-eluting stents (DES) more than one decade ago, different DES have been developed incorporating advances in the metallic mesh, composition and geometry (or elimination) of the polymeric coating, type and dosing of the antiproliferative agent, as well as improvements in the delivery system. Indeed, a number of studies have attested the overall superiority of last-generation DES compared to previous formulations.
As a consequence of modifications in their bioengineering constructs, however, new DES might tend to be more delicate devices, for which maximum care to technical aspects of implantation is of utmost importance to guarantee highest performance. In particular, the utilization of last-generation DES as a workhorse device in the routine practice deserves special attention, since lesion and patient subsets of high complexity are common in this context.
Recently, a new DES formulation built with ultra-thin-strut metallic platform, eluting sirolimus at low doses, abluminal coated with biodegradable polymers, and mounted in a low-compliant delivery system has proven to reduce neointimal proliferation restenosis against bare metal stents in patients of relatively low complexity (1). However, such formulation has not been tested in challenging conditions, such those frequently seen in the daily practice, where patients with adverse morpho-anatomical and biological characteristics are the rule rather than the exception. The present study, therefore, aims to evaluate the performance of the novel DES, when applied to a large population of consecutive patients treated in a busy catheterization laboratory.
Methods
The safety and effectiveness or percutaneous coronary intervention (PCI) performed in our institution is continuously and prospectively assessed by a dynamic registry that captures baseline and follow-up information of selected subsets of interest. The present study population was composed of patients who received at least one Inspiron™ sirolimus-eluting stent (SES) (Scitech, Aparecida de Goiania, Brazil) between April 2013 and January 2015, when this novel device was used as the preferred workhorse stent. There were no specific entry or exclusion criteria and the final decision for the interventional strategy was entirely left at the judgment of the operator. All procedures were performed according to standard techniques. The study was approved by the institutional ethics committee, and written informed consent was obtained from every patient.
The Inspiron stent consists of an ultra-thin (75 µm strut thickness) L-605 cobalt-chromium metallic platform, coated (5 µm thick) with a biodegradable polymer blend of polylactic acid + polylactic-co-glycolic acid exclusively in its abluminal side. The Inspiron elutes sirolimus at low doses [for reference of total drug content in a (2.5-3.0) mm × (18-19) mm stent: Inspiront 84 µg and Cypher™ 125 µg], which is 80% released within 30 days. The study stents were available in diameters from 2.5-3.5 mm and lengths from 12-36 mm. There were no limitations as to the number of stents to be implanted. Biomarkers data (Ck-Mb, troponin or both) were systematically collected in 100% (n=470) of patients after PCI. Angiographic success was defined as residual stenosis <30% by visual analysis in the presence of TIMI 3 grade flow.
Aspirin was initiated before the procedure and maintained lifelong. A loading dose of clopidogrel, prasugrel or ticagrelor was administered for patients not receiving these medications before the procedure. Dual anti-platelet therapy was maintained for 1 year after the procedure.
Baseline, procedural, and follow-up information was prospectively entered into a web-based database. Patients were clinically followed-up at 30 days, 6 months, 1 year and annually thereafter at our out-patient clinic or by telephone call, for those discharged to other institutions. Data from any hospital re-admission was prospectively collected. Patients did not undergo mandatory non-invasive ischemia testing or re-catheterization. The indication for repeat revascularization during follow-up was left at the discretion of the referring team and followed current practice guidelines, being typically triggered by recurrence of chest pain and/or objective evidence of myocardial ischemia. The primary endpoint was the occurrence of major adverse cardiac events (MACE) during follow-up, defined as the composite of cardiac death, non-PCI related myocardial infarction (MI), or target vessel revascularization (TVR). Cardiac death was defined as any death unless unequivocally caused by a non-cardiac cause. MIs were classified as procedure-related and non-procedure-related, as previously described (2). Target lesion revascularization was defined as any repeat intervention to treat the stented segment plus its 5 mm proximal or distal borders. TVR was defined as any repeat revascularization in the epicardial vessel treated in the index procedure. Stent thrombosis were classified by their degree of certainty according to the definition proposed by the Academic Research Consortium (3).
Categorical variable s were presented as percentages and continuous variables were presented as means and standard deviations. The risk of adverse events was estimated using the Kaplan-Meier method. All parameters presented in Tables 1,2 were tested for their predictive value for MACE using Cox regression modelling. Univariate predictors with a P value <0.1 were selected to be tested in a final multivariate model. A P value <0.05 was considered to be significant. Statistical analyses were performed using SPSS version 21.0 (IBM Corporation).
Full table
Full table
Results
Between April 2013 and January 2015, a total of 3247 PCI procedures were performed in 2,572 patients. From the 471 consecutive patients receiving at least one Inspiron™ stent, 470 patients (18.3% from the total PCI procedures within the study period) agreed in participating in the study and comprised the final population.
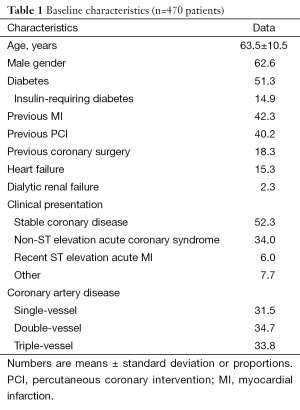
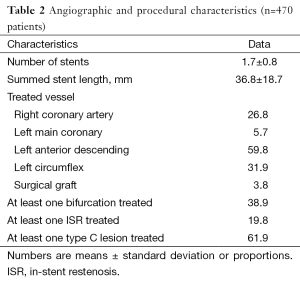
Overall, 51.3% were diabetics, 33.8% had triple-vessel disease, 15.3% had heart failure, 38.9% had at least one bifurcation lesion treated, 19.8% were treated for a bare metal stent restenotic lesion, and 61.9% had at least one type C lesion; one or more of these features were found in 96.0% of the population (Tables 1,2).
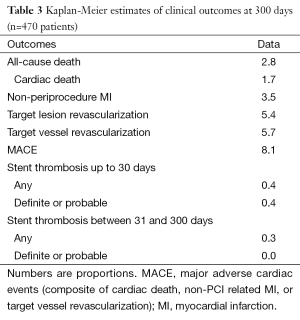
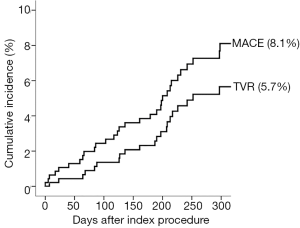
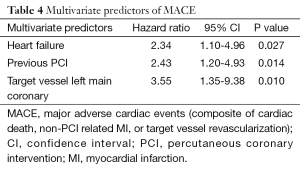
Angiographic success was achieved in 98.73% of the lesions treated with the tested DES. Peri-procedure MI occurred in 4.0% of patients. There were no patients lost and the median follow-up time was 306 days (interquartile range, 208-382 days). At 300 days, 1.7% of patients died due to cardiac causes (Table 3). The incidence of MI as well as the risk of repeat revascularization was low during the follow-up period and the rate of the composite primary endpoint MACE was 8.1% (Table 3 and Figure 1). Overall, 0.7% had any stent thrombosis in the first 300 days, with no cases of definite or probable stent thrombosis between 30 and 300 days (Table 3). Heart failure, previous PCI, and treated left main coronary were identified as multivariate predictors of MACE (Table 4).
Full table
Full table
Discussion
The main finding of the present study is that the tested novel DES had a remarkably good safety and effectiveness profile when utilized to treat patients in the daily practice. It is important to highlight that the study cohort was derived from the daily practice of a busy catheterization laboratory, which utilized the novel DES as a workhorse stent for the most challenging cases. Almost all patients had at least one risk factor for future complications and, nevertheless, target lesion revascularization was approximately 5% at 300 days, with no definite or probable thrombosis detected after 30 days.
The low incidence of adverse events in our population is similar to historical cohorts previously published for last-generation DES (4-7), as illustrated in Table 5. It is noted that when compared to other series (4-7), the high-risk nature of our population emerges unequivocally and reassures the good performance of the tested stent, even in such and unfavourable clinical and angiographic scenario.

Full table
Over the last years, the utility of metallic stents has been confronted against other technologies, namely drug-coated balloons and bioresorbable scaffolds. Paclitaxel-coated balloons have shown efficacious to treat bare metal stent in-stent restenosis (ISR), when compared to first-generation DES or plain balloon dilatation (8). However, more recent data have suggested that paclitaxel-coated may be associated with worse results than last-generation DES (9). Even though previous PCI appeared as an independent predictor of adverse events in the present series, our results points out that the tested stent may be used, with good results, as the device of choice in a population where baseline ISR is a frequent feature.
Notwithstanding the reported utilization of bioresorbable scaffolds in complex patients with relatively good results (10), current on-label recommendations preclude these devices for patients with challenging anatomies (11). The high-risk population that comprises our cohort represents approximately 20% of all patients undergoing PCI during the inclusion period, and would not be ideally treated with vascular scaffolds. The present safety and efficacy results support the usage of DES as the gold-standard devices in complex PCI.
The single-centre nature of the present study may limit its extrapolation to other institutions. Moreover, our study has the intrinsic limitations related to its non-randomized, single-arm, design, since the lack of a control group might hamper a more in-depth analysis of the present findings. Also, few patients were treated after a recent acute MI and none received the stents in the context of primary PCI. Nevertheless, the plethora of information in the literature permitted a fair analysis of our results against the behaviour of other DES and other groups. In fact, the tested stent was associated with undoubtedly promising results, as well as the study cohort appeared as typical population admitted in any busy interventional cardiology department. Final results of a non-inferiority trial comparing the Inspiron stent with the Biomatrix stent are to formally evaluate these two stents in a head-to-head fashion. The reported clinical outcomes were restricted to the first 300 days after the index procedure, which warrants further investigations to inquire the future prognosis in the long run.
Conclusions
Our findings demonstrate that the novel ultrathin-strut, low-dose, SES with abluminal-only biodegradable polymeric coating is associated with excellent short- and mid-term clinical outcomes in patients treated with PCI in the daily practice.
Acknowledgements
The authors are indebted to Mrs. Patricia Pereira and Mrs. Jaqueline R. Silva for their assistance during the conduction of the trial.
Footnote
Conflicts of Interest: Dr. Lemos has received institutional consulting fees and institutional research grants from Scitech and Boston Scientific, institutional educational grants from Medtronic and from Abbott Vascular. Dr Jose Mariani has received institutional consulting fees and research grants from Boston Scientific. Dr. Takimura is a consultant to Scitech. The other authors have no conflicts of interest to declare.
References
- Ribeiro EE, Campos CM, Ribeiro HB, et al. First-in-man randomised comparison of a novel sirolimus-eluting stent with abluminal biodegradable polymer and thin-strut cobalt-chromium alloy: INSPIRON-I trial. EuroIntervention 2014;9:1380-4. [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020-35. [PubMed]
- Serruys PW, Daemen J. Are drug-eluting stents associated with a higher rate of late thrombosis than bare metal stents? Late stent thrombosis: a nuisance in both bare metal and drug-eluting stents. Circulation 2007;115:1433-9; discussion 1439. [PubMed]
- Windecker S, Serruys PW, Wandel S, et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet 2008;372:1163-73. [PubMed]
- Garg S, Wykrzykowska J, Serruys PW, et al. The outcome of bifurcation lesion stenting using a biolimus-eluting stent with a bio-degradable polymer compared to a sirolimus-eluting stent with a durable polymer. EuroIntervention 2011;6:928-35. [PubMed]
- Stefanini GG, Serruys PW, Silber S, et al. The impact of patient and lesion complexity on clinical and angiographic outcomes after revascularization with zotarolimus- and everolimus-eluting stents: a substudy of the RESOLUTE All Comers Trial (a randomized comparison of a zotarolimus-eluting stent with an everolimus-eluting stent for percutaneous coronary intervention). J Am Coll Cardiol 2011;57:2221-32. [PubMed]
- Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet 2013;381:651-60. [PubMed]
- Yu CM, Kwong JS, Sanderson JE. Drug-eluting balloons for coronary artery disease: a meta-analysis of randomized controlled trials. Int J Cardiol 2013;168:197-206. [PubMed]
- Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis: the RIBS V Clinical Trial (Restenosis Intra-stent of Bare Metal Stents: paclitaxel-eluting balloon vs. everolimus-eluting stent). J Am Coll Cardiol 2014;63:1378-86. [PubMed]
- Mattesini A, Secco GG, Dall'Ara G, et al. ABSORB biodegradable stents versus second-generation metal stents: a comparison study of 100 complex lesions treated under OCT guidance. JACC Cardiovasc Interv 2014;7:741-50. [PubMed]
- Wiebe J, Nef HM, Hamm CW. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. J Am Coll Cardiol 2014;64:2541-51. [PubMed]


