Patterns of cardiovascular disease in a group of HIV-infected adults in Yaoundé, Cameroon
Introduction
Human immunodeficiency viral (HIV) infection is a worldwide public health problem with the greatest impact on low income countries (1-3). Increasing access to antiretroviral therapy (ART) has decreased HIV disease associated deaths and increased survival thus making HIV infection a chronic disease (4,5). HIV infection and the metabolic effects associated with ART add to the rising burden of cardiovascular disease in low income countries (6,7). Cardiovascular disease is seen in 30-80% of HIV infected adults, depending on the working definition, screening method, and severity of HIV disease (8). In patients with heart disease, up to 9.7% of patients are infected with HIV (9). All heart structures are involved, and the severity of which correlates with that of immune deficiency (10,11). The pathogenesis of heart disease during HIV infection is multifactorial with the interplay of host cardiovascular risk factors, ART, and HIV infection (12). In the context of increasing availability of ART for patients even at early stage of HIV infection, as deaths due to HIV-associated opportunistic infections are decreasing, increased rates in mortality due to HIV-associated cardiovascular disease are expected among HIV-infected adults. Yet, epidemiological data on cardiovascular disease in HIV-infected individuals are still scare, especially in low-income countries such as Cameroon.
Like other developing countries, Cameroon is experiencing a surge in the prevalence of hypertension, diabetes and other cardiovascular risk factors, and consequential cardiovascular disease (13-16). Ill-prepared health care system, out-of-pocket payments and unaffordable care, and non-optimal management of cardiovascular risk factors by health care providers are major impediments to cope with the rising burden of cardiovascular disease in the country (17-20). Besides, Cameroon is still confronted with a high burden of communicable diseases, especially HIV infection with a national prevalence of 4.3% (2). This work aimed at reporting the baseline clinical, electrocardiographic, and echocardiographic characteristics of cardiovascular disease associated with adult HIV infection in a resource limited setting in Yaoundé, Cameroon.
Methods
Ethical statement
This study was approved by the Institutional Review Board of the Université des Montagnes, ethical clearance N˚ 2014/60/UdM/PR/CAB/CIE. The study was conducted in accordance with the Helsinki declaration.
Study design, setting, and participants
This was a cross-sectional study carried out between February and July 2014 in the Yaoundé Central Hospital and Jamot Hospital which are two teaching hospitals in Yaoundé, the capital city of Cameroon, and a low income setting in sub-Saharan Africa (SSA). Eligible subjects were consenting adults with confirmed HIV infection, aged ≥18 years, presenting with symptoms suggestive of heart disease according to the Framingham clinical diagnostic criteria (21), treated or not treated with antiretroviral medications. Pregnant women and those with known heart disease were excluded.
Procedures
All eligible participants underwent a complete clinical evaluation searching for other elements of cardiovascular disease. We measured resting blood pressures using standardized procedures with the participant in a seated position, and after at least 10 min rest with a mercury sphygmomanometer. The mean of two measures performed at least 3 min apart was used for all analyses. We measured weight in light clothes with a Seca® scale balance to the nearest 0.1 kg, height with a calibrated stadiometer to the nearest 0.5 cm. After 8-12 h overnight fast, blood glucose was measured on total fresh capillary blood samples using the Accu-Chek® Compact Plus glucometer (F. Hoffmann-La Roche AG, Basel, Switzerland), and venous blood samples were drawn from an ante brachial vein for biochemical analyses. Serum lipids including total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol and triglycerides were measured using a Vitros 350 chemistry analyzer (Ortho-Clinical Diagnostics), and CD4 cell counting using a FACS counter. A resting 12-lead electrocardiogram was then performed following standard procedures (speed regulation of 25 mm/s and voltage regulation of 10 mV/10 mm) using a SMART electrocardiograph. A transthoracic cardiac ultrasound was performed with the patient in the left lateral decubitus position by the same cardiologist (APM) using a Philips Sonos 7,500 color Doppler ultrasound machine, initially blinded to the ECG. This was in accordance with the American Society of Echocardiography recommendations (22). A VOLUSON 730 Pro V ultrasound machine with a 50/60 Hz probe was used to study limb vessels in those with suspected deep venous thrombo-embolic (VTE) disease.
Measurements and definitions
Smoking was defined as current tobacco use or use during the last 3 months. Physical activity was defined as at least 150 min of moderate-intensity aerobic physical activity throughout the week or at least 75 min of vigorous-intensity aerobic physical activity throughout the week or an equivalent combination of moderate- and vigorous-intensity activity (23). Patients who did not fulfill this definition were considered physically inactive. We defined hypertension according to World Health Organization recommendation (24) as a resting systolic blood pressure (SBP) ≥140 mmHg and or diastolic blood pressure (DBP) ≥90 mmHg or a patient on antihypertensive treatment. We defined diabetes mellitus as a fasting blood glucose >1.26 g/L at least two time or a patient on antidiabetic treatment (25). We defined overweight as a body mass index (BMI) between 25 and 29.9 kg/m2 and obesity as a BMI ≥30 kg/m2. We defined dyslipidemia as total cholesterol ≥200 mg/L, or triglycerides ≥150 mg/L, or LDLC ≥160 mg/L, or HDLC <40 mg/L in men or <50 mg/L in women. Left ventricular (LV) dilation was defined as indexed LV diameter in diastole >34 mm/m2, and left atrial (LA) enlargement as LA diameter >2.6 mm/m2. Right Ventricular (RV) dilation was defined as RV diastolic area >32 cm2. Pulmonary hypertension (PH) was defined as PAH ≥35 mmHg. Primary PH was considered when there is no evidence of any left heart disease or other possible causes of PH. LV hypertrophy (LVH) was defined as an indexed LV mass (LVMI) >131 g/m2 in men and LVMI >108 g/m2 in women. LV systolic dysfunction was defined as an ejection fraction <45% and RV systolic dysfunction was defined as a Tei myocardial performance index of >0.4. We used Appleton’s criteria to diagnose and classify LV diastolic dysfunction (26). Valves for vegetations, stenosis and insufficiency, global and segmental motions, as well as the presence of intra-cavity thrombus were also studied. The diagnosis of myocarditis was made on clinical arguments of signs of inflammation, electrocardiographic and echocardiographic arguments of low LV ejection fraction, global hypokinesia without dilation of the cavities. No magnetic resonance imaging (MRI) or endomyocardial biopsy was performed in the diagnosis of myocarditis. The diagnosis of dilated cardiomyopathy was made in the presence of low ejection fraction and dilated left ventricle on echocardiography and the absence of any plausible explanation. Ischemic heart disease was diagnosed based on clinical arguments of exertional chest pain, ECG ST segment and T wave changes, and segmental wall motion anomaly on echocardiography. No coronary angiogram was performed as it is not available in our setting. Pericarditis with effusion was made on echocardiography when an echo free space was present between the visceral and parietal pericardium during the entire cardiac cycle. It was considered mild when it measured less than 1 cm, and large when it measured more than 2 cm. No pericardial fluid puncture was made for diagnostic purpose. In our context, this procedure is done only for cases of pericardial tamponade. Tachycardia was defined as a heart rate >100 beats per minute. ECG LVH was defined using Cornell index (RaVL + SV3) >28 mm in men and 20 mm in women. ECG RVH was defined as R/S ratio >1 in V1 or <1 in V5 or V6. Other ECG anomalies studied were repolarization anomalies, presence irregular baseline with irregular rhythm suggestive of atrial fibrillation, or saw tooth baseline suggestive of atrial flutter, premature atrial or ventricular contractions, P wave duration and morphology for atrial enlargement, R wave progression, ST segment abnormalities and presence of S1Q3 wave pattern.
Data analysis
Data were analyzed using Epi Info version 3.5.4 software. Quantitative variables were compared using the Mann-Whitney test, and Pearson’s Chi-squared test of independence was used to study the relationship between qualitative variables. We presented qualitative data as frequencies and proportions, and quantitative data as means with standard deviations (SD). A P value <0.05 was considered statistically significant.
Results
We enrolled 53 HIV-infected patients presenting with symptoms of heart disease, of which 44 were included in data analysis. Nine were lost to follow up after the first visit thus, excluded due to incomplete data. The clinical characteristics are summarized in Table 1. Twenty one (48%) of the patients were men, with an overall mean age of 48.5 (SD 13.1) years (range, 24-72 years). Twenty four patients (54.5%) were diagnosed with HIV for more than 1 year, and 27 (61.4%) had CD4 cell counts <200/mm3. Thirty one (70.5%) patients were on ART, of whom 28 (90.3%) were on first line treatment comprising various combinations of Zidovudine, Lamivudine, Stavudine, Tenofovir, Didanosine, with Nevirapine and Effavirenz. Three patients on second line treatment including first line ART drugs with Lopinavir and Ritonavir. The most frequent cardiovascular risk factors were physical inactivity (61.4%, n=27), hypertension (43.2%, n=19) and dyslipidemia (38.6%, n=17). Hypercholesterolemia, low HDL-cholesterol, hypertriglyceridemia, and high LDL-cholesterol were seen in 8 (18.2%), 11 (25%), 9 (20.5%), and 5 (11.4%) patients respectively. Dyslipidemia was significantly more frequent in ART-treated patients (48.4% vs. 15.4%, P=0.04). The most frequent symptom was exertional dyspnea seen in 38 (86.4%) of patients, with 36.8% in stage II, 26.3% in stage III, and 36.9% in stage IV (New York Heart Association classification). The electrocardiographic and echocardiographic findings are summarized in Table 2. Forty one patients (93.2%) had an abnormal ECG, and the most frequent anomalies were abnormal repolarization seen in 26 (59%) and sinus tachycardia seen in 25 (56.8%) patients. All patients had an abnormal echocardiogram. The most frequent echocardiographic abnormalities were pericardial effusion (46.5%, n=20), and dilated cardiomyopathy (22.7%, n=10). Lower limb venous ultrasound was performed in 11 patients; deep venous thrombosis was seen in 8 (18.2%), which was mainly proximal. There was no right or left predominance in thrombus location. The association of cardiovascular disease findings with CD4 cell counts is summarized in Table 3. Dilated cardiomyopathy was significantly associated with low CD4 cell counts (<200/mm3) (100%, P=0.003). Primary PH only occurred at levels of CD4 cell counts >200/mm3 (100%, P=0.005).
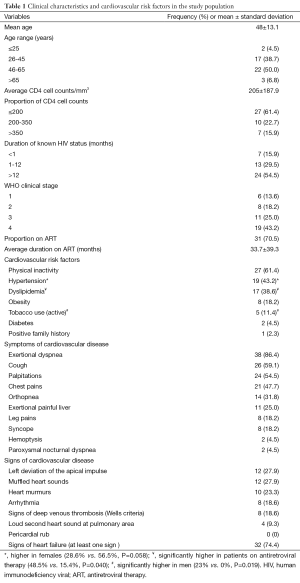
Full table
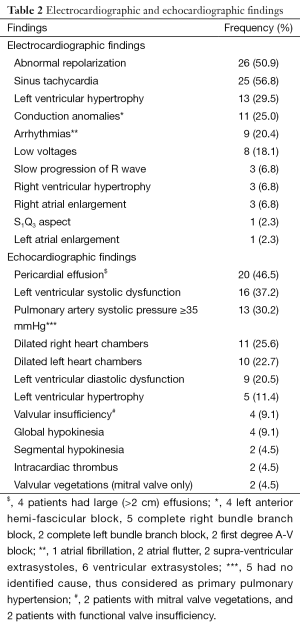
Full table

Full table
Discussion
We found high rates of traditional cardiovascular risk factors and symptomatic cardiovascular disease associated with severe immune deficiency in this young adult population infected with HIV, most of who were on ART for close to three years. The spectrum of cardiac disease in HIV infection varies between studies within and between countries (27). This could be explained by the complex interactions between the traditional cardiovascular risk factors, HIV infection, level of immunity, and antiretroviral treatment. Differences in the methodology used as well as ethnicity could also explain the observed differences. Participants were all young, between the ages of 35 and 45 years, and the most frequently seen conditions were cardiomyopathies, pericardial disease, and PH in varying proportions (6-9,11,27). Other cardiovascular conditions such as coronary heart disease (myocardial infarction in our study) were less frequent and less reported. Valvular heart diseases were not clearly characterized. Common conditions in HIV infection like VTE diseases were less frequently reported.
Few studies in SSA have addressed the problem of classical cardiovascular risk factors in HIV infected adults. Edward et al. reported a moderate to high 10-year cardiovascular risk of 12.8%, predominated by sedentary, and dyslipidemia, with rates similar to our findings (28). Dyslipidemia was frequently seen in those on antiretroviral treatment. Our subjects presented with higher rates of hypertension, obesity, and tobacco use. Smoking rate was lower than that reported by Chillo et al. (29), and it was entirely a male phenomenon as shown by both studies. There could be an under reporting in the rate of tobacco, as its use by women is not a widely accepted practice in SSA. Physical inactivity was very frequent, probably because more than half of the patients had advanced HIV infection and therefore physically and mentally debilitated by ill health. The small sample size of this study could also lead to over estimation of the cardiovascular risk factors.
Cardiomyopathy rates varied widely, ranging from 5% to 57% (12). It has been shown to be associated with worsening immunity, almost exclusively seen when CD4 cell counts are <200/mm3 (11) consistent with our finding. Ayaskanta et al. (11) showed a moderate upward and significant correlation between LV ejection fraction/shortening and CD4 cell counts. This suggests that cardiomyopathy is a marker of severe immune deficiency in HIV disease. The pathogenesis is complex, with interplay of host and viral factors (9,27). It is not clearly known whether improved CD4 cell counts on ART improves on the heart structure and function.
Effusive pericarditis does not seem to correlate with the degree of immune deficiency. It is not significantly associated with low CD4 cell counts <200/mm3 (7,8,29) consistent with our finding. Effusive pericarditis is mainly due to tuberculosis (27).
Primary PH is a frequently reported disease and occurs early in HIV infection (8,30) consistent with our finding. It is associated with poor outcome as specific treatment is economically unavailable to the SSA patient.
VTE disease is under diagnosed and under reported, and it could be responsible for a non-negligible cause of morbidity and mortality in this group of patients. Anzouan-Kacou et al. (8) reported a prevalence rate of 15.5%, slightly lower than our finding, with a small proportion of pulmonary embolism, and Niakara et al. (6) reported a small rate of 3.8%. It was not related to low CD4 cell counts in this study.
The proportion of coronary artery disease (myocardial infarction) was higher in our study compared to that reported by other authors in SSA. It occurred at higher levels of CD4 cell counts. This could be indirectly related to HIV infection via the classical cardiovascular risk factors. Also, the relative small sample size of our study could lead to a relatively higher rate.
This study has some limitations. The cross-sectional and descriptive design does not permit us to establish a cause and effect relation between HIV infection and the observed patterns of heart disease. The small sample size does not give enough power to establish a significant association of HIV infection and some observed disease conditions. The viral load was not measured, which could have contributed to a better understanding of the pathophysiological mechanisms of HIV infection and cardiovascular disorders. Invasive tests to ascertain the diagnosis of myocarditis, pulmonary embolism, and PH were not performed due to insufficient finance to pay for such tests. More so, some of these procedures are not available in this low-income setting. Despite these shortcomings, this study adds to the existing rare information on HIV and cardiovascular diseases in SSA. Patients were prospectively recruited, and all underwent rigorous evaluations and testing using the same instruments and by the same operators, thus ensuring homogeneity and reliability of the collected data.
Conclusions
Cardiovascular risk factors such as hypertension and dyslipidemia are common in HIV-infected adults with heart disease in our milieu. Advanced HIV infection in adults is associated with a high rate of symptomatic heart disease, mostly effusive pericarditis and dilated cardiomyopathy. Primary PH occurred in less advanced HIV disease.
Acknowledgements
We thank all the participants who willingly took part in this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Buba F. Cardiovascular Opportunistic Infections in HIV Disease. Biomedical Research 2011;22:279-84.
- ONUSIDA. Fiche d’information mondiale 2013. Available online: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/20130923_FactSheet_Global_fr.pdf
- UNAIDS. UNAIDS report on the global AIDS epidemic 2013. Available online: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf
- Gandhi RT, Sax PE, Grinspoon SK. Metabolic and cardiovascular complications in HIV-infected patients: new challenges for a new age. J Infect Dis 2012;205:S353-4. [PubMed]
- Oramasionwu CU, Hunter JM, Brown CM, et al. Cardiovascular Disease in Blacks with HIV/AIDS in the United States: A Systematic Review of the Literature. Open AIDS J 2012;6:29-35. [PubMed]
- Niakara A, Drabo YJ, Kambire Y, et al. Cardiovascular diseases and HIV infection: study of 79 cases at the National Hospital of Ouagadougou (Burkina Faso). Bull Soc Pathol Exot 2002;95:23-6. [PubMed]
- Nzuobontane D, Blackett KN, Kuaban C. Cardiac involvement in HIV infected people in Yaoundé, Cameroon. Postgrad Med J 2002;78:678-81. [PubMed]
- Anzouan-kacou JB, Dogoua P, Konin C, et al. Affections cardio-vasculaires chez les patients à sérologie VIH positive non traités par anti-rétroviraux. Cardiovascular diseases in HIV patients not receving antiretroviral therapy. Cardiologie tropicale 2013;131:1-7.
- Sliwa K, Carrington MJ, Becker A, et al. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J 2012;33:866-74. [PubMed]
- Ntusi NB, Taylor D, Naidoo NG, et al. Progressive human immunodeficiency virus-associated vasculopathy: time to revise antiretroviral therapy guidelines? Cardiovasc J Afr 2011;22:197-200. [PubMed]
- Ayaskanta S, Sidhartha D, Rabindra KD. Study of Cardiac Manifestations in Patients with HIV Infection and Their Correlation with CD4 Count in Indian Population. Int J Clin Med 2012;3:178-83.
- Currier JS. Update on cardiovascular complications in HIV infection. Top HIV Med 2009;17:98-103. [PubMed]
- Kingue S, Ngoe CN, Menanga AP, et al. Prevalence and Risk Factors of Hypertension in Urban Areas of Cameroon: A Nationwide Population-Based Cross-Sectional Study. J Clin Hypertens (Greenwich) 2015;17:819-24. [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas. 6th edition. Brussels: International Diabetes Federation; 2013.
- Jingi AM, Noubiap JJ, Kamdem P, et al. The spectrum of cardiac disease in the West Region of Cameroon: a hospital-based cross-sectional study. Int Arch Med 2013;6:44. [PubMed]
- Jingi AM, Noubiap JJ, Yonta EW, et al. A Centre for the Diagnosis and Treatment of Tuberculosis (CDT) in a resource-limited setting: a dragnet for patients with heart disease? Arch Public Health 2014;72:26. [PubMed]
- Noubiap JJ, Joko WY, Obama JM, et al. Community-based health insurance knowledge, concern, preferences, and financial planning for health care among informal sector workers in a health district of Douala, Cameroon. Pan Afr Med J 2013;16:17. [PubMed]
- Jingi AM, Noubiap JJ, Ewane Onana A, et al. Access to diagnostic tests and essential medicines for cardiovascular diseases and diabetes care: cost, availability and affordability in the West Region of Cameroon. PLoS One 2014;9:e111812. [PubMed]
- Noubiap JJ, Jingi AM, Veigne SW, et al. Approach to hypertension among primary care physicians in the West Region of Cameroon: substantial room for improvement. Cardiovasc Diagn Ther 2014;4:357-64. [PubMed]
- Jingi AM, Nansseu JR, Noubiap JJ. Primary care physicians' practice regarding diabetes mellitus diagnosis, evaluation and management in the West region of Cameroon. BMC Endocr Disord 2015;15:18. [PubMed]
- McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441-6. [PubMed]
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440-63. [PubMed]
- World Health Organization. Physical activity and adults: recommended levels of physical activity for adults 18-64 years. Available online: http://www.who.int/dietphysicalactivity/factsheet_adults/en/
- 1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 1999;17:151-83. [PubMed]
- American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care 2011;34:S11-61. [PubMed]
- Appleton CP, Firstenberg MS, Garcia MJ, et al. The echo-Doppler evaluation of left ventricular diastolic function. A current perspective. Cardiol Clin 2000;18:513-46. ix. [PubMed]
- Syed FF, Sani MU. Recent advances in HIV-associated cardiovascular diseases in Africa. Heart 2013;99:1146-53. [PubMed]
- Edward AO, Oladayo AA, Omolola AS, et al. Prevalence of traditional cardiovascular risk factors and evaluation of cardiovascular risk using three risk equations in nigerians living with human immunodeficiency virus. N Am J Med Sci 2013;5:680-8. [PubMed]
- Chillo P, Bakari M, Lwakatare J. Echocardiographic diagnoses in HIV-infected patients presenting with cardiac symptoms at Muhimbili National Hospital in Dar es Salaam, Tanzania. Cardiovasc J Afr 2012;23:90-7. [PubMed]
- Cicalini S, Almodovar S, Grilli E, et al. Pulmonary hypertension and human immunodeficiency virus infection: epidemiology, pathogenesis, and clinical approach. Clin Microbiol Infect 2011;17:25-33. [PubMed]


