
Lung transplantation for pediatric pulmonary arterial hypertension—quo vadis?
Introduction
End-stage pulmonary arterial hypertension (PAH) in children continues to have a high mortality despite improving medical therapy with 5-year survivals ranging between 57–75% depending on the etiology of PAH (1).
In this scenario, lung or combined heart/lung transplantation remains the only curative therapy for patients with either idiopathic or associated pulmonary hypertension following congenital heart defects. However, transplantation as a therapeutic strategy involves multiple aspects that must be considered individually for each patient. This includes the patient’s individual course of disease, implicating the urgency of the transplantation as well as serious comorbidities such as end-organ failure, history of malignancy and psychosocial aspects involving the entire family and social support system of the patient.
In pediatric patients, timing of listing for transplantation is especially challenging, given the longer prospective waiting times for a suitable donor organ and the desire to postpone transplantation and systemic immunosuppression as long as possible whilst maintaining adequate end-organ function.
Experience and knowledge on the rather small pediatric patient cohort derives primarily from small, retrospective studies and case series rendering definite therapeutic recommendations regarding timing of listing for transplantation difficult.
This review focuses on current practice in pediatric lung transplantation for pulmonary arterial hypertension, including timing of referral to a transplant center, listing criteria as well as surgical techniques and postoperative management including new developments in extracorporeal circulation and outcomes of lung transplantation in pediatric patients.
Worldwide experience with pediatric lung transplantation for PAH
Pediatric lung transplantation remains a rare medical therapy, with an average of ~100 annual registered transplants worldwide in the ISHLT database in the past decade (2). Starting from the 1990s, a transition from combined heart/lung transplantations to bilateral lung transplantations for PAH took place in pediatric as well as adult patients, leading to better overall survival (5-year survival 53% vs. 79% for pediatric patients) (2). Above that, that trend allowed for allocation of more donor hearts towards (pediatric) recipients of isolated hearts. Between 2002 and 2018, a total of only 174 pediatric lung transplantations for idiopathic PAH and 75 transplants for non-idiopathic PAH were reported to the ISHLT registry (2).
Of the almost 40 transplant centers reporting pediatric lung transplantation activity, the vast majority (~90%) of transplants was performed in centers with an annual pediatric transplant volume of less than five transplants (3). Therefore, referral of pediatric patients with pulmonary arterial hypertension to high-volume centers with sufficient experience in treating this challenging sub-cohort in pediatric lung transplantation is recommended (3). This is especially important, since waiting list mortality for pediatric patients appears to be higher as compared to most adults in lung transplantation (4,5), and has been reported of being 8–13 deaths per 100 patient-years for pediatric lung transplantation in the US in the current era (6).
Lung or combined heart/lung transplantation?
The decision whether to list a patient for single or bilateral lung transplantation, or alternatively combined heart/lung transplantation, is generally made with regards to the underlying disease as well as anatomical considerations. Until the early 1990s, patients with the underlying diagnosis of pulmonary hypertension mostly underwent combined heart/lung transplantation, however, knowledge on the cardiac ability to remodel following bilateral lung transplantation has led to the now common practice of preferring bilateral lung transplantation in patients with pulmonary arterial hypertension (7). Cardiac remodeling occurs early after lung transplantation due to the decreased right ventricular afterload as well as the elevated left ventricular volume preload due to the healthy lung vasculature and parenchyma of the allograft (8).
Therefore, pediatric patients with pulmonary arterial hypertension in the absence of irreversible structural cardiac defects nowadays receive bilateral lung transplantations. This includes most children with high-grade tricuspid valve regurgitation, which generally improves quickly following lung transplantation. Children with congenital cardiac defects should be evaluated individually with regard to the underlying heart defect and the prospective hemodynamic changes of the cardiac defect after lung transplantation. In general, combined heart/lung transplantation in pediatric patients nowadays is rare and primarily a therapeutic option for children with Eisenmenger’s disease and irreparable heart disease (9).
In pediatric lung transplantation, pulmonary arterial hypertension and other pulmonary vascular diseases are the leading diagnoses in recipients <5 years. These diagnoses include idiopathic as well as associated pulmonary arterial hypertension due to developmental lung diseases such as alveolar capillary dysplasia, bronchopulmonary dysplasia, and congenital diaphragmatic hernia as well as pulmonary vascular diseases such as pulmonary veno-occlusive disease or pulmonary capillary hemangiomatosis.
Of note, single lung transplantation for PAH has a significantly worse short- and long-term survival as compared to bilateral lung transplantation (2).
Listing criteria for transplantation
The decision of when to admit a pediatric patient to the waiting list remains difficult, since waiting time with regard to body height, total lung capacity and blood group is not as predictable in children as it is in adults.
Generally, evaluation for transplantation should be initiated before episodes of severe cardiac decompensation with simultaneous end-organ failure occur. Timing is certainly difficult and expert consensus guidelines state no clear recommendation on when lung transplantation must be considered within the course of disease. The dynamics of disease progression appear to be the most valuable tool when considering active placement on the waiting list, with rapid progression of symptoms over time indicating a higher benefit from immediate listing and transplantation (10).
Retrospective cohort analyses repeatedly identified unresponsiveness to maximal medical combination therapy and persisting WHO functional class III and IV as a good prognostic parameter, suggesting that these patients benefit from transplantation (11,12). Additional aspects that might be considered when listing for lung transplantation in children include failure to thrive and echocardiographic and/or invasive hemodynamic assessments indicating low cardiac output or severe right ventricular dysfunction. All of the above have been recognized of being associated with higher risks of death in pediatric PAH patients (1). Similarly, an increase of serum NT-proBNP has been recognized in a meta-analysis of being a sensitive prognostic marker for pediatric pulmonary arterial hypertension (12) and serum levels of >1,200 pg/mL in children >1 year of age have been included as a high-risk determinant for death in the 2019 consensus statement on pediatric pulmonary hypertension (10). Thus, the course of NT-proBNP over time appears to be a helpful tool when evaluating potential transplant candidates.
Recurrent cardiac syncopes, indicating intermittent right ventricular failure, have also been determined a high-risk factor for death in pediatric PAH patients, suggesting emergent evaluation for transplantation.
Furthermore, events of life-threatening hemoptysis in pediatric PAH patients due to progression of the underlying disease is associated with a poor outcome, indicating urgent need for active listing for lung transplantation (13).
In relatively stable children with slowly but constantly progressing disease, initiation of intravenous or subcutaneous prostacyclin treatment has been used in the past as an indicator that discussing listing for transplantation might be due. In an era of changing paradigms of PH treatment and a more proactive management with early use of combination therapy, which may stabilize the patient and slow down disease progression, other more objective criteria are paramount. Other listing strategies may exist, depending on organ allocation systems, e.g., in countries, where accrued waiting time on an active list is expedient.
Unfortunately, in the clinical real world, the authors have experienced many pediatric PAH patients ultimately undergoing lung transplantation to already require extracorporeal membrane oxygenation support or intensive care treatment at the time of listing for lung transplantation. This stresses the concern that timely listing for transplantation is more often missed than achieved and reconfirms that early referral to the transplant center remains important for patients at a high risk for hemodynamic deterioration.
Addressing this topic early, sharing the facts with the family and—if adequate with the patient—is crucial for the counselling process. Early familiarization with this option, timely referral and work-up may reduce stress levels and allows a considerate decision, rather than transplant teams being forced to act under pressure on limited information in a critical condition of a child.
Contraindications for lung transplantation
The general contraindications for lung transplantation apply for pediatric patients with PAH, too. Importantly, these include a history of malignancy within the past 5 years, with only a few exceptions.
An important further contraindication to consider for patients with PAH is end-organ dysfunction other than lung- (and heart-)failure such as renal failure or liver impairment due to chronic right heart failure. In general, chronic renal failure due to low cardiac output syndrome will most likely recover after lung transplantation, however, patients are already on hemodialysis prior to transplantation will most likely suffer from sustained renal impairment and may require dialysis even after transplantation. Since combined lung-kidney transplantation has shown poor results in the past, a staged approach with primary lung transplantation and secondary living-related kidney transplantation a few months after lung transplantation may be considered in pediatric patients.
A more serious contraindication appears to be advanced liver impairment due to chronic congestion. Liver fibrosis may be reversible after normalization of right heart hemodynamics, however, liver cirrhosis (“cirrhose cardiaque”) has to be regarded a contraindication for lung transplantation. Combined lung-liver transplantation may be considered in experienced centers; however, this procedure has rarely been successfully performed for PAH as underlying disease, thus outcomes remain questionable.
The psychosocial background of children and their families must be evaluated prior to lung transplantation with respect to therapy adherence including compliance to pharmacotherapy and social support.
Infection or colonization with specific bacteria species contraindicating lung transplantation are uncommon in pediatric PAH cohorts.
Relative contraindications for lung transplantation in PAH children importantly include measures of surgical complexity and critical condition (ICU treatment) of the recipient. Surgical complexity is mainly determined by previous cardiac and thoracic procedures implicating severe adhesions, bleeding and therefore sometimes multiple surgical revisions weighing into the postoperative course. Critical condition may include secondary organ dysfunction (of the kidney and/or liver), vasopressor requirement, mechanical ventilation, sepsis, coma, sedation and ECMO. While each of these relative contraindications might easily be overcome by an otherwise well-suited transplant candidate, combinations of these relative contraindications might as well constitute a prohibitive risk. For example, the combination of previous thoracic surgeries and bridging on ECMO requiring anticoagulation easily leads to life-threatening bleeding during or early after lung transplant surgery severely impairing transplant outcomes.
Bridging options to lung transplantation
Hemodynamic decompensation due to acute (on chronic) pulmonary hypertension with right heart failure may occur in patients during the course of the underlying disease due to acute increase of right ventricular afterload. In children requiring treatment on an intensive care unit due to hemodynamic decompensation, invasive hemodynamic support is usually considered when conservative measures such as diuretics or inotropic support in addition to intravenous prostanoid therapy fail to stabilize the patient.
Children compensate severe hemodynamic impairment in every-day life better than adults and severe decompensations with the need for extracorporeal life support often occur unexpected. The authors’ experience in a high-volume pediatric lung transplant program shows, that approx. 24% of pediatric PAH patients require ECMO support prior to lung transplantation and 48% of all pediatric PAH patients are admitted to an intensive care unit before transplantation. These high proportions indicate the sudden dramatic deterioration of this patient cohort.
In general, early identification of patients with severe hemodynamic compromise and immediate initiation of therapeutic measures including veno-arterial extracorporeal membrane oxygenation dramatically improve the prognosis of these children (14,15). Acute hemodynamic decompensation in patients with PAH is a life-threatening emergency with high risk of cardiopulmonary resuscitation, which is especially challenging in this patient cohort given the severely elevated pulmonary vascular resistance.
Importantly, patients with severe pulmonary arterial hypertension are at high risk for cardiopulmonary collapse and resuscitation in the course of all medical procedures including initiation of anesthesia or placement of central lines in awake patients. It is therefore advisable to have emergency veno-arterial ECMO available for decompensating PAH patients eligible for lung transplantation.
Extracorporeal membrane oxygenation
Pediatric patients in acute right heart failure without sufficient response to medical therapy can be bridged to lung transplantation using extracorporeal circulation. Given the pathophysiology of the underlying disease, severe hemodynamic limitation with low cardiac output syndrome is usually the leading symptom, thus implantation of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is frequently required in this patient cohort. As a rule of thumb, kidney failure requiring hemodialysis and/or noradrenaline rate at >0.2 µg/kg/min indicate a necessity of VA-ECMO implant. Depending on age and body weight, cannulas for VA-ECMO may be placed in the femoral vessels (16). In small children <20 kg, central cannulation is necessary requiring sternotomy or clam-shell access. In newborns and infants <2 years of age, while being only infrequently candidates for lung transplantation, VA-ECMO is initiated via carotid artery cannulation. VA-ECMO allows cardiorespiratory stabilization in patients with decompensated PAH by decreasing RV preload and increasing systemic perfusion with oxygenated blood, thus restoring end-organ function. In this setting, venous blood is withdrawn from the right atrium through a cannula into the ECMO, which consists of an oxygenator and a pump. The oxygenated and decarboxylated blood is then returned to the patients ascending aorta, carotid or femoral artery depending on the cannulation mode. Therefore, VA-ECMO can support and even fully replace heart and lung function in patients with PAH.
ECMO systems have improved considerably over the past two decades, with currently available ECMO circuits possessing better hemocompatibility, and having reduced complications from bleeding while requiring less anticoagulation as compared to systems available in the 1990s (17).
In patients with simultaneous or exclusive oxygenation deficits, veno-venous extracorporeal membrane oxygenation (VV-ECMO) can improve clinical symptoms either alone or in combination with VA-ECMO, resulting in veno-veno-arterial ECMO (VVA-ECMO). However, hypoxia as the leading cause for clinical deterioration without right heart impairment in patients with decompensated PAH is uncommon and VV-ECMO hardly ever is the right choice for PAH patients.
An alternative approach to ECMO are pumpless lung assist devices, in which the cannulas are implanted parallel to the lung into the main pulmonary artery and left atrium, blood flow being generated through the disease-related high PA pressures. Several transplant programs published successful bridging of PAH patients to lung transplantation including adolescent patients (18-20), yet, VA-ECMO has proven superior, since implementation can be performed without thoracotomy in most patients via femoral cannulation, hemodynamic support is provided independent of the patients cardiac performance and the ECMO circuit can be continuously used during transplantation as cardiopulmonary support (21).
Another important aspect for bridging to successful lung transplantation is the concept of awake ECMO, in which patients on extracorporeal life support independent of cannulation or ECMO mode are awake and spontaneously breathing. Bridging on awake ECMO has been shown to result in improved outcomes post lung transplantation as compared to sedation and mechanical ventilation in adult as well as pediatric patients. This strategy has been successfully used in children <1 year of age and older, by extubation immediately after initial sedation for ECMO implantation (22-27).
Reversed Potts shunt
Two centers published their experience on performing a Potts shunt as a rescue maneuver in patients with acute heart failure due to acute on chronic pulmonary arterial pressure. Reversed Potts Shunts are surgical or transcatheter shunts created between the left pulmonary artery and the descending aorta, effectively leading to a pulmonary artery pressure drainage, thereby decreasing right ventricular afterload. Given the hemodynamic changes of the blood flow, the shunt is a therapeutic option only for patients with suprasystemic PA pressures and systemic cyanosis of the lower body half is inevitable. Since the venous blood is drained into the descending aorta, myocardial and cerebral hypoxia is generally avoided.
Using this invasive strategy in order to achieve clinical improvement of the patients was the proposed strategy in most reports, however, bridge to transplant or as a therapeutic tool to defer transplantations have been reported as well.
The St. Louis group reported their experience of Potts shunts in children with PAH in a small series of 5 cases with an age range between 4–16 years. Of the five children, two did not benefit from surgery. No successful lung transplant of this cohort has been performed thus far and the team refers to another patient that underwent lung transplantation with a Potts shunt in situ, that died of fatal hemorrhage from the chest wall during transplant surgery. Given this severe complication, the team proposes a new technique for better control of the shunt itself during transplantation, in which a ligature with a snare is left in situ allowing closure of the shunt during transplantation, a so far theoretical manoeuvre (4,28).
Paradela et al. published their multi-center experience of using Potts shunts as a palliative therapy tool in 24 pediatric patients with drug-refractory PAH. Overall, three patients died early due to low cardiac output and one patient died 2 years after shunt establishment. The remaining 20 children showed postinterventional clinical improvement of symptoms including better WHO functional class and improving right heart function. One patient required lung transplantation 6 years after Potts shunt surgery and the shunt was closed in an interventional procedure immediately before transplantation in order to allow sufficient cardiopulmonary bypass circulation. Transplantation and postoperative course of this patient remained uneventful (29).
In conclusion, Potts shunts can ameliorate clinical symptoms in pediatric patients with suprasystemic PA pressures, however, sufficient data on outcomes following lung transplantation in these patients is lacking.
Donor selection and donor pool expansion
Suitable donors for pediatric lung transplantation are scarce, since size-matching is of utmost importance in lung transplantation surgery and pediatric donors are rare. Thus, waiting times may last months or years depending on region and allocation system. In addition, pediatric donor utilization for lung transplantation remains low between 19–35% in the United States and Europe, suggesting that acceptance of donor organs can be improved (30).
Allocation is generally led by blood-group, age, body height and weight as well as predicted total lung capacity (pTLC). In general, mild oversizing of the allograft (Donor-Recipient pTLC ratio 1.2:1) appears to have a favorable outcome in adult lung transplantation, however, no data on pediatric lung transplantation is available defining the ideal donor-recipient pTLC ratio (31).
Lobar transplantation and graft size reduction
For children and adolescents with small chest cavities for whom time on the waiting list might be unacceptable long, acceptance of a larger donor graft suitable for size-reduced lobar lung transplantation has been reported to be an alternative with good postoperative short- and long-term outcome.
The Zurich group reported their institutional experience in pediatric lung transplantation, where 69% of the children received size-reduced donor grafts with comparable postoperative outcome to non-size reduced lung transplantations (32).
These data being in line with an older study from the Hannover group, who, at the time performed 57% of pediatric lung transplantation as size-reduced grafts without major postoperative limitations in the recipient cohort as compared to recipients of full-sized donor organs (33).
Similar findings were published by Marasco et al., finding comparable postoperative outcomes in 23 patients receiving lobar grafts (34).
Thus, performing anatomical lobar or atypical size reduction of donor allografts for pediatric recipients is feasible without compromising postoperative short- and long-term outcome. This strategy potentially alleviates the donor organ shortage and can in urgent pediatric patients reduce waiting list time and mortality by using adult organs (35).
The majority of high-volume transplant centers preferably uses both lower lobes when performing bilateral lobar transplantation, since the shape of the lower lobes more closely resembles the shape of whole lungs. However, if the lower lobes show parenchymal damage in the donor, upper lobes can be used likewise (36). In practical terms, donor lungs with pTLC ratios >1.5, if accepted at all, should be considered for atypical (middle lobe and lingula) resection, pTLC ratios of >2.5 would mandate (lower) lobar transplants and even higher ratios could lead to consideration of upper lobar transplants, given the upper lobes are usually slightly smaller than the lower lobes.
However, the risk of reperfusion edema with severe primary graft dysfunction, postoperative renal failure and longer intensive care unit stay is higher in lobar lung transplant recipients as demonstrated by the Pittsburgh group (37), a circumstance that appears to be even more important in PAH patients, given the higher PA pressures during initial reperfusion as compared to other underlying diseases. While mandatory utilization of extracorporeal circulation in lung transplantation for severe PAH attenuates this phenomenon, the Hannover group has largely avoided performing lobar or size reduced lung transplants for severe PAH given the additional risk of complications.
Donation after cardiac death (DCD)
Outside Germany, the need to enlarge the donor pool has led transplant surgeons to use controlled donors after cardiac death (DCD Maastricht category III) for routine lung transplantation with excellent postoperative outcome in adults in those countries permitting DCD donation. In pediatric lung transplantation, experience with DCD donors is limited. Pawale et al. first reported on a 10-year-old child receiving single-lung transplantation for pulmonary vein stenosis following pneumectomy of the contralateral side with suprasystemic pulmonary pressure prior to lung transplantation. The child received a right-sided single-lung transplantation from a 13-year-old donor who died due to anoxic brain damage following traumatic asphyxiation. Graft function and recovery of the recipient were uneventful, suggesting that utilization of DCD organs in pediatric lung transplantation is similarly feasible as it is in adults (38).
In line with this single case report is data from the Melbourne group, summarizing their experience on using DCD donors for pediatric recipients. A total of nine children underwent lung transplantation using DCD donor lungs, of which 4 organs were procured from pediatric donors. One-year survival in this group was 100%, suggesting that DCD in pediatric lung transplantation provides good donor lung quality. In total, the group has utilized fifteen pediatric DCD lung donors in their program (39).
Extended criteria donor lungs
The dramatic donor shortage in the majority of western countries has been widely discussed and led to an increasing use of non-ideal donor organs in lung transplantation. Previously defined ideal donor organ criteria included age <55 years, PaO2/FiO2 ratio >300 mmHg, as well as a clear chest X-ray, absence of chest trauma, sterile broncho-alveolar lavage fluid and the absence of purulent endobronchial secretions. In adult lung transplantation, organs not meeting these criteria have been extensively and increasingly used by large transplant centers over the past two decades without impairing long-term outcomes as compared to standard donors (40).
Whilst using non-standard donor organs for lung transplantation, a suitable donor-recipient risk matching has been found to be an important strategy to maintain good outcomes in lung transplantation. Selecting low-risk recipients for extended criteria donor lungs and in return using standard donor organs for high-risk recipients such as patients being bridged to transplant on extracorporeal life support led to similarly good postoperative outcomes (41).
In pediatric lung transplantation, similar results were obtained by the Hannover group, finding no significant difference in postoperative survival and CLAD-free survival when utilizing non-standard donor organs for pediatric lung transplantation. Of note, 5 pediatric recipients with the underlying disease PAH in this analysis received non-standard donor lungs (30).
However, lung transplantation for PAH as underlying disease itself is generally categorized as a procedure with elevated risk, thus an overall good donor organ quality remains recommended.
ABO incompatible lung transplantation
ABO-incompatible lung transplantation can be performed in pediatric patients <2 years of age due to their immature immune system and absence of ABO-antibodies. ABO-incompatible pediatric heart transplantation has been performed for decades with comparable long-term results to ABO-compatible transplants (42). Large centers have adopted this technique for pediatric lung transplantation with protocols typically including plasma exchange using extracorporeal circulation during transplantation surgery and repetitive monitoring of isohemagglutinin levels (43,44).
Of note, ABO-incompatible lung transplantation can be performed in older patients with acceptable outcome, however, perioperative management is significantly more complex making it nonpractical for routine use (45).
Living-donor lobar lung transplantation (LDLLT)
LDLLT is a feasible treatment option, in which two donors donate one lung lobe each for one recipient receiving bilateral lobar transplantation. Given these complex circumstances, LDLLT is an option only for a highly-selected patient cohort since two ABO-compatible healthy donors must be available with an acceptable size-compatibility between two donors and the recipient.
Although the first LDLLTs were initially performed in the United States as a measure to reduce waiting list mortality in times of cadaveric donor scarcity, the largest series in LDLLT has repeatedly been reported from Japan over the past years (46).
The group reported their experience of 11 recipients with the underlying disease PAH prior to LDLLT in 2007, of which 4 patients were children <18 years old. Donors were exclusively 1st degree relatives and spouses, who donated one lower lung lobe each (47).
Size matching in LDLLT is critical, leading the group around H. Date to the development of a formula for calculation of total Forced Vital Capacity of the two donor grafts:
Total FVC of both donor grafts = measured FVC of the right donor × 5/19 + measured FVC of the left donor × 4/19 [1]
The authors describe the donor-recipient matching as acceptable, when total FVC of the 2 donor grafts was >50% of the predicted FVC of the recipient regardless of underlying disease (48).
Within the cohort of 11 patients receiving LDLLT for PAH, 5-year survival was 100% with no mortality or relevant morbidity in the donors. Of note, two pediatric patients developed chronic lung allograft dysfunction on one side, the remaining contralateral allograft being unaffected, suggesting protective side-effects in living-related lung transplantation due to a potential immunological privilege.
Surgical technique and perioperative considerations
Pediatric lung transplantation in PAH patients carries unique surgical challenges that derive from the special hemodynamic situation in anatomically small children and the requirement of extracorporeal circulation during transplantation surgery.
Surgical techniques for lung transplantation have been described elsewhere (4), however, the specific surgical approach for pediatric lung transplantation in PAH may deviate slightly from standard lung transplantation techniques.
In general, induction of anesthesia in PAH patients is associated with a high risk of cardiopulmonary decompensation and resuscitation (49), requiring ECMO stand-by during this phase. Ventilation during surgery should routinely include nitric oxide, reducing the risk of low cardiac output and hemodynamic deterioration (50).
Extracorporeal membrane oxygenation has been shown superior for hemodynamic support during lung transplantation as compared to conventional cardiopulmonary bypass by multiple centers, also applying to pediatric lung transplantation for PAH and other underlying diseases (51-53).
Cardiopulmonary bypass remains necessary only if a simultaneous cardiac procedure—such as an ASD closure—is planned.
In general, veno-arterial ECMO is utilized with femoral access in children >20 kg with open surgical dissection of the femoral vessels in order to avoid vascular complications and establishment of a distal leg perfusion using a size-appropriate sheath. Central cannulation is used in recipients <20 kg, since femoral vessels are likely not of an acceptable size for cannulation (54).
In small children requiring central ECMO cannulation, a clamshell thoracotomy is usually the preferred surgical approach, whereas in children >20 kg with femoral ECMO cannulation, bilateral anterolateral thoracotomies are performed.
Surgical sequence in lung transplantation for pulmonary arterial hypertension includes performing all intrathoracic surgical dissection prior to pneumectomy off extracorporeal circulation and anticoagulation for prevention of postoperative bleeding complications, this being of special importance in patients with adhesions due to prior thoracic surgeries (55).
Immediately prior to ECMO implantation, an unfractionated Heparin bolus of 100 IU/kg body weight is administered for anticoagulation. Of note, the authors abstain from measuring activated clotting time and no repetitive doses of heparin are administered throughout surgery since the ECMO circuit and cannulas are heparin coated and clotting of the ECMO circuit during surgery has never been experienced by the authors.
The technique of lung transplantation surgery in pediatric recipients includes anastomoses of the main bronchi using resorbable polydioxanone running sutures for the pars membranacea and single stitches of the cartilage rings. The anastomoses are sealed with fibrin glue mixed with gentamycin and vancomycin after bronchoscopic assessment of airway patency before performing the vascular anastomosis. Accurate estimation of the length of donor and recipient pulmonary arteries is important in order to avoid kinking of the artery. In PAH recipients, diameters of the native vessels are usually larger than the donor PA diameter, requiring a consistent distribution of stitches throughout the running suture with an adequate proportion of donor to recipient vessel progress.
The venous anastomosis between the left atrium of the recipient and the pulmonary veins is usually performed with an everting suture between both left atrial myocardial cuffs, this technique potentially decreasing thromboembolic complications. Of note, vascular anastomoses in pediatric patients are routinely performed using resorbable polydioxanone sutures to avoid vascular stricture during growth.
Postoperative management
Postoperative ECMO treatment for LV remodeling
In PAH patients, VA-ECMO is routinely left in place after transplant surgery as a prophylactic measure against primary graft failure. More precisely, primary graft failure following lung transplantation for severe PAH most of the time really is lung edema caused by diastolic dysfunction of the chronically under filled left ventricle. VA-ECMO provides the support needed to allow for left ventricular remodeling following the altered hemodynamic conditions after lung transplantation (8,56,57).
Therefore, patients having undergone lung transplantation for severe PAH receive a left atrial catheter prior to chest closure for monitoring of the LA pressure during ECMO treatment and weaning. Continuous postoperative VA-ECMO treatment prevents ‘flooding’ of the left ventricle, that would lead to left ventricular dysfunction due to volume overload with subsequent pulmonary congestion. Therefore, VA-ECMO treatment in PAH patients is continued for a minimum of 5 days after transplantation and weaning is conducted with close monitoring of left atrial pressure and echocardiographic observation of left ventricular end-diastolic diameter (LVEDD). No anticoagulation is administered within the first 48 hours after surgery to prevent postoperative bleeding.
Of extraordinary importance in lung transplantation is fast weaning off mechanical ventilation, in the case of patients with severe PAH as underlying disease whilst still being on ECMO support, since spontaneous breathing reduces the risk of infectious complications and muscular strength of the recipient is preserved, avoiding the need for long-term mechanical ventilation. This approach is feasible even in very young children with central ECMO cannulation. Of note, in children with femoral cannulation, an additional heparin line connected to the distal leg perfusion sheath is advisable due to the small diameter of the catheter.
ECMO explantation is performed once LA pressure remains 10 mmHg or less and LVEDD does not increase upon ECMO flow reduction, suggesting that the left ventricle is capable of handling full cardiac output without dilatation and functional impairment.
In children with femoral vessel cannulation, ECMO explantation usually requires surgical insertion of a patch into the femoral artery to avoid arterial stenosis. This procedure can be performed with local anesthesia in the spontaneously breathing pediatric recipient, minimizing the risk of reintubation after lung transplantation.
Pharmacotherapy following lung transplantation
Standard triple immunosuppression in most lung transplant programs currently includes steroids, mycophenolate mofetil and tacrolimus (2).
Induction therapy with either an anti-thymocyte globulin (ATG) or an IL2-receptor antagonist is performed in about 74% of programs reporting to the ISHLT database (2). The authors’ program does not routinely use induction therapy in lung transplantation with comparable outcome results as compared to published ISHLT data (2,41).
In addition, lung transplant recipients require cytomegaly virus (CMV) prophylaxis, usually with ganciclovir or valganciclovir, as well as anti-fungal prophylaxis, usually with itraconazole or voriconazole/posaconazole in patients colonized with aspergillus sp., in the first 3 to 6 postoperative months. Above that, a perioperative broad-spectrum antibiotic treatment covering both gram-positive and -negative pathogens is recommended throughout the phase of postoperative ECMO treatment.
In addition, life-long prophylaxis for Pneumocystis jirovecii infection using cotrimoxazole (twice weekly) is required.
Outcome of pediatric lung transplantation for PAH
According to the international (ISHLT) database, survival after pediatric lung transplantation for IPAH is comparable to pediatric lung transplants for other underlying diseases with a 1-year survival of approx. 80% and a 5-year survival of 64–65%. At Hannover Medical School, where standard postoperative ECMO treatment has been routinely performed over years, 1-year survival (95.2%) in pediatric PAH recipients is superior, indicating the significance of LV remodeling after lung transplantation in PAH patients (Figure 1). These results are comparable to adult lung transplantation for IPAH, however, outcome of pediatric lung transplantation for PAH other than idiopathic PAH is inferior as compared to IPAH recipients (median survival 3.2 vs. 7.4 years) (2).
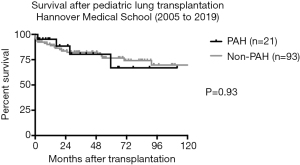
Long-term postoperative morbidity is primarily attributed to chronic rejection, BOS-free survival at 5 years post-transplant being 45.7% in pediatric recipients (58).
Incidence of other morbidities including severe renal dysfunction (creatinine >2.5mg/dL) at 5 years is lower in pediatric patients (5.9%) as compared to adults (16%) in the recent era since 2005. In pediatric lung transplant recipients, 9.3% develop malignancies within 5 years after transplantation with lymphoma being the leading disease (2).
Outlook
Given that survival at 5 years post pediatric lung transplantation exceeds 80% in experienced centers and evolving techniques in the field of lung transplantation allow greater utilization of the existing donor pool and the future prospect of personalized immunosuppression may also improve outcome following lung transplantation, survival prospects in children with end-stage pulmonary vascular disease are currently as good as never before.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Christian Apitz and Astrid Lammers) for the series “Pediatric Pulmonary Hypertension” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/cdt-21-65). The series “Pediatric Pulmonary Hypertension” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ivy DD, Abman SH, Barst RJ, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol 2013;62:D117-26. [Crossref] [PubMed]
- Hayes D Jr, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-second pediatric lung and heart-lung transplantation report-2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant 2019;38:1015-27. [Crossref] [PubMed]
- Sweet SC. Pediatric Lung Transplantation. Respir Care 2017;62:776-98. [Crossref] [PubMed]
- Lancaster TS, Eghtesady P. State of the Art in Pediatric Lung Transplantation. Semin Thorac Cardiovasc Surg 2018;30:166-74. [Crossref] [PubMed]
- OPTN/SRTR 2017 Annual Data Report: Lung. Available online: https://srtr.transplant.hrsa.gov/annual_reports/2017/Lung.aspx
- Tsuang WM, Chan KM, Skeans MA, et al. Broader Geographic Sharing of Pediatric Donor Lungs Improves Pediatric Access to Transplant. Am J Transplant 2016;16:930-7. [Crossref] [PubMed]
- Schaellibaum G, Lammers AE, Faro A, et al. Bilateral lung transplantation for pediatric idiopathic pulmonary arterial hypertension: a multi-center experience. Pediatr Pulmonol 2011;46:1121-7. [Crossref] [PubMed]
- Tudorache I, Sommer W, Kühn C, et al. Lung transplantation for severe pulmonary hypertension--awake extracorporeal membrane oxygenation for postoperative left ventricular remodelling. Transplantation 2015;99:451-8. [Crossref] [PubMed]
- Hayes D Jr, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-second pediatric lung and heart-lung transplantation report-2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant 2019;38:1015-27. [Crossref] [PubMed]
- Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant 2019;38:879-901. [Crossref] [PubMed]
- Hansmann G, Apitz C. The Need for Comprehensive Cardiac Catheterization in Children With Pulmonary Hypertension. J Am Coll Cardiol 2016;67:1009-10. [Crossref] [PubMed]
- Ploegstra MJ, Arjaans S, Zijlstra WMH, et al. Clinical Worsening as Composite Study End Point in Pediatric Pulmonary Arterial Hypertension. Chest 2015;148:655-66. [Crossref] [PubMed]
- Roofthooft MT, Douwes JM, Vrijlandt EJ, et al. Frequency and prognostic significance of hemoptysis in pediatric pulmonary arterial hypertension. Am J Cardiol 2013;112:1505-9. [Crossref] [PubMed]
- Paden ML, Conrad SA, Rycus PT, et al. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J 2013;59:202-10. [Crossref] [PubMed]
- Sivarajan VB, Almodovar MC, Rodefeld MD, et al. Pediatric extracorporeal life support in specialized situations. Pediatr Crit Care Med 2013;14:S51-61. [Crossref] [PubMed]
- Casswell GK, Pilcher DV, Martin RS, et al. Buying time: The use of extracorporeal membrane oxygenation as a bridge to lung transplantation in pediatric patients. Pediatr Transplant 2013;17:E182-8. [Crossref] [PubMed]
- Tagarakis GI, Tsilimingas NB. Heparin-coated extracorporeal circulation systems in heart surgery. Recent Pat Cardiovasc Drug Discov 2009;4:177-9. [Crossref] [PubMed]
- Strueber M, Hoeper MM, Fischer S, et al. Bridge to thoracic organ transplantation in patients with pulmonary arterial hypertension using a pumpless lung assist device. Am J Transplant 2009;9:853-7. [Crossref] [PubMed]
- Mayes J, Niranjan G, Dark J, et al. Bridging to lung transplantation for severe pulmonary hypertension using dual central Novalung lung assist devices. Interact Cardiovasc Thorac Surg 2016;22:677-8. [Crossref] [PubMed]
- Patil NP, Mohite PN, Reed A, et al. Modified technique using Novalung as bridge to transplant in pulmonary hypertension. Ann Thorac Surg 2015;99:719-21. [Crossref] [PubMed]
- Olsen MC, Anderson MJ, Fehr JJ, et al. ECMO for Pediatric Lung Transplantation. ASAIO J 2017;63:e77-80. [Crossref] [PubMed]
- Schmidt F, Sasse M, Boehne M, et al. Concept of "awake venovenous extracorporeal membrane oxygenation" in pediatric patients awaiting lung transplantation. Pediatr Transplant 2013;17:224-30. [Crossref] [PubMed]
- Schmidt F, Jack T, Sasse M, et al. "Awake Veno-arterial Extracorporeal Membrane Oxygenation" in Pediatric Cardiogenic Shock: A Single-Center Experience. Pediatr Cardiol 2015;36:1647-56. [Crossref] [PubMed]
- Ius F, Natanov R, Salman J, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation may not impact overall mortality risk after transplantation: results from a 7-year single-centre experience. Eur J Cardiothorac Surg 2018;54:334-40. [Crossref] [PubMed]
- Biscotti M, Gannon WD, Agerstrand C, et al. Awake Extracorporeal Membrane Oxygenation as Bridge to Lung Transplantation: A 9-Year Experience. Ann Thorac Surg 2017;104:412-9. [Crossref] [PubMed]
- Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8. [Crossref] [PubMed]
- Olsson KM, Simon A, Strueber M, et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant 2010;10:2173-8. [Crossref] [PubMed]
- Grady RM, Eghtesady P. Potts Shunt and Pediatric Pulmonary Hypertension: What We Have Learned. Ann Thorac Surg 2016;101:1539-43. [Crossref] [PubMed]
- Paradela M, Mercier O, Baruteau A, et al. Endovascular closure of Potts shunt before double lung transplantation for idiopathic pulmonary arterial hypertension. J Thorac Cardiovasc Surg 2013;146:e5-7. [Crossref] [PubMed]
- Sommer W, Ius F, Müller C, et al. Extended criteria donor lungs do not impact recipient outcomes in pediatric transplantation. J Heart Lung Transplant 2019;38:560-9. [Crossref] [PubMed]
- Eberlein M, Diehl E, Bolukbas S, et al. An oversized allograft is associated with improved survival after lung transplantation for idiopathic pulmonary arterial hypertension. J Heart Lung Transplant 2013;32:1172-8. [Crossref] [PubMed]
- Schmid FA, Inci I, Bürgi U, et al. Favorable outcome of children and adolescents undergoing lung transplantation at a European adult center in the new era. Pediatr Pulmonol 2016;51:1222-8. [Crossref] [PubMed]
- Mueller C, Hansen G, Ballmann M, et al. Size reduction of donor organs in pediatric lung transplantation. Pediatr Transplant 2010;14:364-8. [Crossref] [PubMed]
- Marasco SF, Than S, Keating D, et al. Cadaveric lobar lung transplantation: technical aspects. Ann Thorac Surg 2012;93:1836-42. [Crossref] [PubMed]
- Aigner C, Winkler G, Jaksch P, et al. Size-reduced lung transplantation: an advanced operative strategy to alleviate donor organ shortage. Transplant Proc 2004;36:2801-5. [Crossref] [PubMed]
- Deuse T, Sill B, von Samson P, et al. Surgical technique of lower lobe lung transplantation. Ann Thorac Surg 2011;92:e39-42. [Crossref] [PubMed]
- Mahesh B, Bhama JK, Odell DD, et al. Surgical Strategy for Lung Transplantation in Adults With Small Chests: Lobar Transplant Versus a Pediatric Donor. Transplantation 2016;100:2693-8. [Crossref] [PubMed]
- Pawale A, McKean M, Dark J, et al. Successful pediatric single-lung transplantation with previous contralateral pneumonectomy, using controlled "donation after cardiac death" lung, for congenital pulmonary vein stenosis. J Thorac Cardiovasc Surg 2010;139:e125-6. [Crossref] [PubMed]
- Snell G, Levvey B, Paraskeva M, et al. Controlled donation after circulatory death (DCD) donors: A focus on the utilization of pediatric donors and outcomes after lung transplantation. J Heart Lung Transplant 2019;38:1089-96. [Crossref] [PubMed]
- Sundaresan S, Semenkovich J, Ochoa L, et al. Successful outcome of lung transplantation is not compromised by the use of marginal donor lungs. J Thorac Cardiovasc Surg 1995;109:1075-9; discussion 1079-80. [Crossref] [PubMed]
- Sommer W, Kühn C, Tudorache I, et al. Extended criteria donor lungs and clinical outcome: results of an alternative allocation algorithm. J Heart Lung Transplant 2013;32:1065-72. [Crossref] [PubMed]
- Urschel S, West LJ. ABO-incompatible heart transplantation. Curr Opin Pediatr 2016;28:613-9. [Crossref] [PubMed]
- Grasemann H, de Perrot M, Bendiak GN, et al. ABO-incompatible lung transplantation in an infant. Am J Transplant 2012;12:779-81. [Crossref] [PubMed]
- Carlens J, Müller C, Tudorache I, et al. ABO-Incompatible Infant Lung Transplantation - Report on First Two Cases from Germany. Montreal: Annual Meeting International Society for Heart and Lung Transplantation, 2020: abstract 1484.
- Strüber M, Warnecke G, Hafer C, et al. Intentional ABO-incompatible lung transplantation. Am J Transplant 2008;8:2476-8. [Crossref] [PubMed]
- Date H. Living-related lung transplantation. J Thorac Dis 2017;9:3362-71. [Crossref] [PubMed]
- Date H, Kusano KF, Matsubara H, et al. Living-donor lobar lung transplantation for pulmonary arterial hypertension after failure of epoprostenol therapy. J Am Coll Cardiol 2007;50:523-7. [Crossref] [PubMed]
- Date H, Aoe M, Nagahiro I, et al. Living-donor lobar lung transplantation for various lung diseases. J Thorac Cardiovasc Surg 2003;126:476-81. [Crossref] [PubMed]
- Latham GJ, Yung D. Current understanding and perioperative management of pediatric pulmonary hypertension. Paediatr Anaesth 2019;29:441-56. [Crossref] [PubMed]
- Green JB, Hart B, Cornett EM, et al. Pulmonary Vasodilators and Anesthesia Considerations. Anesthesiol Clin 2017;35:221-32. [Crossref] [PubMed]
- Ius F, Sommer W, Tudorache I, et al. Five-year experience with intraoperative extracorporeal membrane oxygenation in lung transplantation: Indications and midterm results. J Heart Lung Transplant 2016;35:49-58. [Crossref] [PubMed]
- Ius F, Kuehn C, Tudorache I, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg 2012;144:1510-6. [Crossref] [PubMed]
- Aigner C, Wisser W, Taghavi S, et al. Institutional experience with extracorporeal membrane oxygenation in lung transplantation. Eur J Cardiothorac Surg 2007;31:468-73; discussion 473-4. [Crossref] [PubMed]
- Kaufeld T, Beckmann E, Ius F, et al. Risk factors for critical limb ischemia in patients undergoing femoral cannulation for venoarterial extracorporeal membrane oxygenation: Is distal limb perfusion a mandatory approach? Perfusion 2019;34:453-9. [Crossref] [PubMed]
- Sommer W, Ius F, Kühn C, et al. Technique and Outcomes of Less Invasive Lung Retransplantation. Transplantation 2018;102:530-7. [Crossref] [PubMed]
- Salman J, Ius F, Sommer W, et al. Mid-term results of bilateral lung transplant with postoperatively extended intraoperative extracorporeal membrane oxygenation for severe pulmonary hypertension. Eur J Cardiothorac Surg 2017;52:163-70. [Crossref] [PubMed]
- Moser B, Jaksch P, Taghavi S, et al. Lung transplantation for idiopathic pulmonary arterial hypertension on intraoperative and postoperatively prolonged extracorporeal membrane oxygenation provides optimally controlled reperfusion and excellent outcome. Eur J Cardiothorac Surg 2018;53:178-85. [Crossref] [PubMed]
- Lammers AE, Burch M, Benden C, et al. Lung transplantation in children with idiopathic pulmonary arterial hypertension. Pediatr Pulmonol 2010;45:263-9. [Crossref] [PubMed]


