
Coronary computed tomography angiography-based assessment of vascular inflammation in patients with obstructive sleep apnoea and coronary artery disease
Introduction
Obstructive sleep apnoea (OSA) has been associated with an increased burden of coronary artery disease (CAD) while severe OSA has been associated with high plaque burden (1). Repeated fluctuations to airflow characteristic of OSA result in intermittent hypoxia that drives systemic vascular inflammation and oxidative stress (2). Vascular inflammation within the coronary arteries is particularly associated with increased plaque vulnerability and the risk of acute coronary syndrome (ACS) (3,4). Serum biomarkers of vascular inflammation, however, offer poor localisation to the coronary vasculature and demonstrate significant heterogeneity in the context of OSA (2,5). Inflammation induces localised phenotypic changes in subcutaneous and visceral adipose tissue depots that are quantifiable on coronary computed tomography angiography (CTA), and therein presents an alternative means of assessing inflammatory risk (6). Subcutaneous adipose tissue (SCAT) attenuation on CT serves as a marker of systemic inflammation (6,7). Epicardial adipose tissue (EAT) is enclosed within the visceral pericardium and is contiguous with the coronary vessels (8), and both volumetric and attenuation-based measurements of EAT have been associated with coronary inflammation and atherosclerotic plaque (9,10). Pericoronary adipose tissue (PCAT) is a specific subset of EAT including only adipocytes directly adjacent to the coronary vasculature, and is an emerging biomarker of coronary inflammation in patients with CAD (11,12). Growing evidence suggests pro-inflammatory adipose tissue changes may be present in OSA (13,14), but the extent to which these changes are observable in the systemic vasculature compared to the coronary arteries specifically within patients with OSA has yet to be explored. Moreover, it is unclear if the severity of OSA exhibits an additive effect to the increased inflammatory profile observed in patients with a high coronary plaque burden (15). Our study aimed to evaluate the association of coronary CTA-derived markers in SCAT, EAT and PCAT with severe OSA and coronary plaque burden in patients with OSA.
We present the following article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-338/rc).
Methods
Patients
Data was obtained retrospectively from adult patients with a clinically indicated overnight polysomnography (PSG) study within 12 months of coronary CTA for suspected CAD between 2011 to 2017. Type 1 PSG studies were conducted under laboratory conditions, while type 2 studies involved a portable full PSG monitor that may be used by patients in the home. Both types of PSG study provide reliable clinical assessment of OSA (16) and were included in our analysis. Only PSG studies with a diagnostic recording period of ≥2 hours, the minimum duration for reliable diagnosis of severe OSA, were included (17). Clinical measurements and adjudication of sleep studies were completed according to American Academy of Sleep Medicine (AASM) guidelines. AASM 2007 alternate criteria (18) were used for 39 sleep studies before 2015, and AASM 2012 recommended criteria (19) were used for the remaining 52 studies after this time. Patient characteristics were collected at the time of PSG or coronary CTA. Body mass index (BMI) measurements were typically taken at the time of PSG. Traditional cardiovascular risk factors (hypertension, hyperlipidaemia, smoking status and diabetes) and current treatment with statins were obtained at the time of coronary CTA. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Research Ethics Committee of Monash Health (No. EC00382) and individual consent for this retrospective analysis was waived.
OSA classification
OSA presence was defined according to the apnoea/hypopnea index (AHI), per AASM guidelines described previously (18,19). Data pertaining to respiratory events, arousal and levels of oxygen desaturation were all collected as part of PSG studies in either the lab or home environment as discussed. OSA presence was defined as AHI ≥5 events per hour. Mild OSA was defined as AHI 5–14, moderate OSA as AHI 15–29, and severe OSA as AHI >30 events per hour.
Severe OSA and high plaque burden classification
As stated, severe OSA was defined as AHI >30, and a high plaque burden was defined as CT-adapted Leaman score (CT-LeSc) ≥8.3, while a low plaque burden was defined as CT-LeSc <8.3. Patients with both severe OSA and high plaque burden were classified as Group 1, while patients with only severe OSA, only high plaque burden, or neither severe OSA nor high plaque burden were classified as Group 2.
Coronary CTA protocol
All coronary CTA acquisitions were obtained using a 320-detector row scanner (Aquilion Prime; Canon Medical Systems Corporation, Otawara, Japan), obtained via prospective electrocardiogram (ECG)-triggering and during a single-breath-hold, with a heart rate of 60 bpm maintained through use of beta-blockers. All images studied were contrast-enhanced using Omnipaque 350 (60–90 mL) administered intravenously at a rate of 5 mL/s. Acquisition parameters consisted of tube current 300–500 mA, tube voltage 100–120 kV, collimation 320 mm ×0.5 mm, gantry rotation time 275 ms and temporal resolution 175 ms. Images were collated and reconstructed using FC43 reconstruction kernel and adaptive iterative dose-reduction three-dimensional algorithm (AIDR3D, Canon Medical Systems).
Plaque classification
All clinical coronary CTA scans were adjudicated for plaque by two cardiologists simultaneously with at least 2 years of experience in coronary CTA. Plaque burden was quantified using the CT-LeSc, which provides a numeric scoring system denoting severity of coronary plaque adjusted for vessel localisation and dominance, degree of stenosis (obstructive versus non-obstructive) and type of plaque (calcified versus non-calcified or mixed plaque). High plaque burden was defined as CT-LeSc ≥8.3 as described previously (20). Additionally, the prevalence of plaque calcification, defined as calcified or mixed plaque detected on coronary CTA, was studied.
Adipose tissue analysis
Three adipose tissue depots were assessed independently using semi-automated software: SCAT, EAT and PCAT. Adipose tissue volume for all depots was measured in cm3 and attenuation was measured in Hounsfield units (HU). Adipose tissue was automatically computed by software as three-dimensional voxels between −190 to −30 HU on contrast-enhanced slices, within the specific volumes of interest delineated manually for each depot. Adipose tissue analyses were performed by two operators with at least 1 year of experience with semi-automated coronary CTA analysis (J Yuvaraj and W Cameron).
Subcutaneous adipose tissue attenuation (SCAT-a) was assessed using QFAT (version 2.5, Cedars-Sinai Medical Center, Los Angeles, CA, USA) by manual contouring of two 1-mm2 circular region of interest (ROI) markers at two slices corresponding to distinct anatomical landmarks: the pulmonary bifurcation and the left main coronary artery (21). The average attenuation within each ROI was determined, and the per-patient value for SCAT was calculated using the average of all four ROIs. Epicardial adipose tissue volume (EAT-v) and attenuation (EAT-a) were likewise computed using the same software. Both EAT-v and EAT-a were quantified as adipose tissue located between the manually-traced visceral pericardium and the automatically-detected epicardial surface of the heart, extending from an upper slice of the pulmonary bifurcation to a lower slice of the appearance of the posterior descending artery (22). PCAT attenuation (PCAT-a) was assessed using AutoPlaque (version 2.5), around the proximal right coronary artery. PCAT-a was quantified automatically as adipose tissue within concentric radial layers extending up to 3 mm orthogonally from the semi-automatically contoured outer coronary wall, within a 40-mm longitudinal segment beginning 10 mm from the ostium (12). This was used as a representative biomarker of global coronary inflammation.
Statistical analysis
Data distributions were assessed for normality. Measures of central tendency and spread varied depending on normality: parametric data was described in terms of mean and standard deviation; and for non-parametric data median and interquartile range (IQR) were used. Comparative analysis of categorical covariates was performed using the Chi-square test or the Fisher exact test when cell values were less than five. Comparison of differences in a continuous covariate across two categories was performed using the independent t-test for parametric data, and the Mann-Whitney U test for non-parametric data. Comparison of two continuous variables was performed via either Pearson or Spearman correlation analysis for parametric and non-parametric data, respectively. Analysis of association of cardiovascular risk factors and the presence of severe OSA and high plaque burden with EAT-a and PCAT-a was performed using univariable and multivariable linear regression analyses. Only covariates achieving a P<0.2 on univariable analysis were included in multivariable models, while a two-sided P<0.05 was considered significant on multivariable analysis. All statistical analysis was performed using SPSS (version 26).
Results
Baseline patient characteristics are summarised in Table 1. A total of 91 patients were studied (mean age: 59.3±11.1 years; 71.4% male). Median AHI was 20.0 (IQR, 11.1 to 44.5). Most patients had OSA (90.1%, n=82). Of patients with OSA, 32.9% had mild OSA (n=27), 26.8% had moderate OSA (n=22), and 41.5% had severe OSA (n=34). AHI was correlated with CT-LeSc (r=0.24, P=0.023). Median AHI was significantly higher in patients with a high plaque burden than in patients with a low plaque burden [34.3 (IQR, 18.9 to 56.0) vs. 15.8 (IQR, 6.9 to 33.6), P<0.001]. High plaque burden was significantly more prevalent in patients with severe OSA than in those with mild-moderate OSA [52.9% (n=18) vs. 28.1% (n=16), P=0.018]. No significant differences in prevalence of traditional risk factors for CAD were found among patients with both severe OSA and high plaque burden (Table 1).
Table 1
| Characteristics | OSA | Group | |||||
|---|---|---|---|---|---|---|---|
| Severe (n=34) | Mild-moderate (n=57) | P value | 1 (n=18) | 2 (n=73) | P value | ||
| Age, mean ± SD | 58.8±10.9 | 59.6±11.3 | 0.752 | 60.9±10.7 | 58.9 ± 11.2 | 0.494 | |
| Male sex, n (%) | 26 (76.5) | 39 (68.4) | 0.478 | 16 (88.9) | 49 (67.2) | 0.067 | |
| AHI, median (IQR) | 56.0 (41.4, 73.3) | 12.5 (7.5, 19.0) | <0.001 | 53.7 (38.4, 64.7) | 16.1 (9.7, 25.2) | <0.001 | |
| CT-LeSc, median (IQR) | 9.0 (3.0, 12.2) | 5.4 (0.0, 9.0) | 0.090 | 11.5 (10.0, 16.9) | 4.6 (0.0, 7.6) | <0.001 | |
| CT-LeSc >8.3 | 18 (52.9) | 16 (28.1) | 0.018 | – | – | – | |
| Plaque calcification, n (%) | 20 (58.8) | 27 (47.4) | 0.290 | 14 (77.8) | 33 (45.2) | 0.017 | |
| Statin, n (%) | 17 (50.0) | 20 (35.1) | 0.161 | 9 (50.0) | 28 (38.4) | 0.368 | |
| Cardiovascular risk factors, n (%) | |||||||
| Hypertension | 19 (55.9) | 33 (57.9) | 0.851 | 10 (55.6) | 42 (57.5) | 0.879 | |
| Hyperlipidaemia | 18 (52.9) | 29 (50.9) | 0.849 | 10 (55.6) | 37 (50.7) | 0.711 | |
| Diabetes | 8 (23.5) | 12 (21.1) | 0.783 | 4 (22.2) | 16 (21.9) | 0.978 | |
| Smoker | 7 (20.6) | 5 (8.8) | 0.107 | 1 (5.6) | 11 (15.1) | 0.285 | |
| Family history of CAD | 14 (41.2) | 23 (40.4) | 0.938 | 9 (50.0) | 28 (38.4) | 0.368 | |
| Obesity | 25 (73.5) | 32 (56.1) | 0.097 | 11 (61.1) | 46 (63.0) | 0.881 | |
OSA, obstructive sleep apnoea; SD, standard deviation; AHI, apnoea/hypopnoea index; IQR, interquartile range; CT-LeSc, computed tomography-adapted Leaman score; CAD, coronary artery disease.
Adipose tissue volume in severe OSA and high plaque burden
Differences in adipose tissue volume in Group 1 and Group 2 are shown in Table 2. Median EAT-v in all patients was 80.0 cm3 (IQR, 48.5 to 116.0 cm3). EAT-v was numerically higher but not statistically significant in patients with severe OSA and high plaque burden [103.5 (IQR, 50.0 to 135.0) vs. 73.0 (IQR, 48.5 to 109.5) cm3, P=0.139]. PCAT-v, likewise, was numerically higher but not statistically significant in patients with severe OSA and high plaque burden [1.53 (IQR, 1.32 to 2.00) vs. 1.49 (IQR, 1.18 to 1.74) cm3, P=0.094].
Table 2
| Variables | Group 1 | Group 2 | P value |
|---|---|---|---|
| Attenuation (HU) | |||
| SCAT-a | −107.6 (−118.8, −100.3) | −108.4 (−117.8, −103.1) | 0.939 |
| EAT-a | −87.6 (−90.8, −84.0) | −84.0 (−87.1, −80.0) | 0.011 |
| PCAT-a | −90.4 (−94.9, −86.1) | −83.4 (−90.9, −79.0) | 0.008 |
| Volume (cm3) | |||
| EAT-v | 103.5 (50.0, 135.0) | 73.0 (48.5, 109.5) | 0.139 |
| PCAT-v | 1.53 (1.32, 2.00) | 1.49 (1.18, 1.74) | 0.094 |
All values are described as median (IQR). OSA, obstructive sleep apnoea; HU, Hounsfield units; SCAT-a, subcutaneous adipose tissue attenuation; EAT-a, epicardial adipose tissue volume; PCAT-a, pericoronary adipose tissue attenuation; EAT-v, epicardial adipose tissue volume; PCAT-v, pericoronary adipose tissue volume; IQR, interquartile range.
Adipose tissue attenuation in severe OSA and high plaque burden
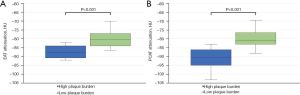
Differences in adipose tissue attenuation in patients with severe OSA and high plaque burden are shown in Table 2. Median SCAT-a in all patients was −108.1 HU (IQR, −117.8 to −103.0 HU). SCAT-a was not significantly different between Group 1 and Group 2 [−107.6 (IQR, −118.8 to −100.3) vs. −108.4 (IQR, −117.8 to −103.1) HU, P=0.939]. In all patients, median EAT-a was −84.0 HU (IQR, −88.0 to −81.0), and median PCAT-a was −85.7 HU (IQR, −92.4 to −79.6 HU). Both EAT-a and PCAT-a were significantly lower in Group 1 than in Group 2 [EAT-a: −87.6 (IQR, −90.8 to −84.0) vs. −84.0 (IQR, −87.1 to −80.0) HU, P=0.011 (Figure 1A); PCAT-a: −90.4 (IQR, −94.9 to −86.1) vs. −83.4 (IQR, −90.9 to −79.0) HU, P=0.008 (Figure 1B)].

To investigate the relationship between plaque burden and severe OSA, we evaluated EAT-a and PCAT-a in patients with severe OSA and high plaque burden compared to patients with severe OSA and low plaque burden. Patients with severe OSA and high plaque burden had significantly lower EAT-a and PCAT-a than patients with severe OSA but low plaque burden [EAT-a: −87.6 (IQR, −90.8 to −84.0) vs. −80.3 (IQR, −84.0 to −77.0) HU, P<0.001 (Figure 2A); PCAT-a: −90.4 (IQR, −94.9 to −86.1) vs. −80.9 (IQR, −83.1 to −76.5) HU, P<0.001 (Figure 2B)]. Additionally, patients with severe OSA and high plaque burden had a higher prevalence of plaque calcification compared to patients with severe OSA and low plaque burden [77.8% (n=14) vs. 37.5% (n=6), P=0.017]. However, the prevalence of either statin use or obesity did not significantly differ between these two subgroups (both P>0.05).
We further explored differences in EAT-a and PCAT-a relative to OSA severity independent of significant coronary plaque burden, that is, in patients with only a low coronary plaque burden. Patients with low plaque burden and severe OSA had significantly higher EAT-a compared to patients with low plaque burden and mild-moderate OSA [−80.3 (IQR, −84.0 to 77.0) vs. −84.0 (IQR, −87.1 to −81.0) HU, P=0.020 (Figure 3A)]. Patients with low plaque burden and severe OSA had numerically, but not significantly, higher PCAT-a in comparison to patients with low plaque burden and mild-moderate OSA [−80.9 (IQR, −83.1 to −76.5) vs. −83.8 (IQR, −90.7 to −79.0) HU, P=0.113 (Figure 3B)]. Between these subgroups there were no significant differences in the prevalence of plaque calcification, statin use and obesity (all P>0.05).

Multivariable analysis
Multivariable linear regression analyses used to assess EAT-a and PCAT-a as outcome variables are summarised in Table 3. Severe OSA and high plaque burden, and hypertension, were included in multivariable analyses. EAT-a was independently associated with severe OSA and high plaque burden. PCAT-a was associated with severe OSA and high plaque burden, and with hypertension.
Table 3
| Variables | EAT-a | PCAT-a | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | ||||||||||||
| Beta | 95% CI | P value | Beta | 95% CI | P value | Beta | 95% CI | P value | Beta | 95% CI | P value | ||||
| Severe OSA and high plaque burden | −3.5 | −6.6, −0.3 | 0.031 | −3.4 | −6.5, −0.3 | 0.022 | −5.8 | −10.8, −0.8 | 0.024 | −6.0 | −10.8, −1.2 | 0.016 | |||
| Hypertension | −1.8 | −4.3, −0.8 | 0.168 | −1.7 | −4.2, 0.8 | 0.176 | −5.5 | −9.5, −1.4 | 0.009 | −5.6 | −9.5, −1.6 | 0.006 | |||
| Hyperlipidaemia | −0.3 | −2.8, 2.3 | 0.847 | – | – | – | 0.5 | −3.7, 4.7 | 0.805 | – | – | – | |||
| Diabetes | −0.02 | −3.0, 3.0 | 0.992 | – | – | – | −1.7 | −6.9, 3.5 | 0.513 | – | – | – | |||
| Smoker | 1.4 | −2.5, 5.3 | 0.466 | – | – | – | 3.4 | −2.6, 9.5 | 0.257 | – | – | – | |||
EAT, epicardial adipose tissue; PCAT, pericoronary adipose tissue; OSA, obstructive sleep apnoea; CI, confidence interval.
Discussion
In our study we have sought to evaluate adipose tissue volume and density in patients with severe OSA and significant coronary plaque burden. Firstly, we confirm previous findings that the severity of OSA is associated with an increased coronary plaque burden (1,23,24). Moreover, we report that patients with both severe OSA and a high coronary plaque burden harbour decreased attenuation of both EAT and PCAT compared to patients with either mild-moderate OSA or a low plaque burden. However, when controlling for the effect of significant coronary plaque, the attenuation of both EAT and PCAT is increased in patients with severe OSA compared to mild-moderate OSA, suggesting a unique inflammatory phenotype induced by OSA independent of the influence of CAD.
White adipose tissue as a marker of inflammatory risk
The effects of intermittent hypoxia, a key pathophysiological feature in OSA, have been shown to drive increases in vascular inflammatory biomarkers (2,14,25). Despite ample evidence denoting the plausibility of increased vascular inflammation in OSA, studies evaluating inflammatory biomarkers in humans continue to demonstrate significant heterogeneity (5). Moreover, treatment with the first-line therapy for symptomatic OSA in continuous positive airway pressure (CPAP) has been shown to produce variable results, with a reduction in inflammatory biomarkers found among OSA patients (2,26,27) but little change in patients with both OSA and CAD (28), emphasising a need to consider the interplay between OSA and other cardiovascular disease states that may themselves be associated with vascular inflammation. Associations between increased epicardial fat mass and OSA have been shown previously (29-32), but there is a paucity of literature evaluating CT characteristics of epicardial fat in patients with the combination of severe OSA alongside significant coronary disease. We report decreased attenuation of both EAT and PCAT in patients with both severe OSA and a high plaque burden, which may correspond to the accumulation of lipid-rich ‘white’ adipose tissue (WAT), although this needs to be adjusted for EAT volume and BMI, which have been associated with decreased PCAT attenuation (33). Adipocyte hypertrophy that would accompany increased EAT mass is associated with an enhanced pro-inflammatory milieu characterised by the heightened secretion of pro-inflammatory adipokines (34), and accordingly WAT accumulation facilitates a chronic low-grade inflammatory state. The impact of adipokines on vasculature is well established and indicates an ‘outside-inside’ signalling relationship, in which adipocytes beyond the vessel wall can elicit a pro-inflammatory response within the vasculature (35). However, the nature of the inverse ‘inside-outside’ signalling in OSA remains unclear, that is, whether vascular inflammation arising from recurrent intermittent hypoxia may also induce the accumulation of low attenuation WAT or other changes to cardiac adipose tissue. Gozal et al. (36) found that in mice, intermittent hypoxia exposure induces WAT accumulation and macrophage infiltration in adipose tissue, whereas sustained hypoxia, bereft of the temporary periods of reoxygenation seen in intermittent hypoxia, results in an atheroprotective ‘brown’ adipose tissue (BAT) phenotype. Nevertheless, determining the nature of this ‘inside-outside’ signalling among OSA patients with comorbidities would provide clarity as to the degree that adipose tissue attenuation may reflect changes representative of an intravascular inflammatory state specific to OSA.
Impact of coronary calcification on adipose tissue attenuation
Decreased adipose tissue attenuation may indeed correspond to WAT hypertrophy due to lipid accumulation, but the degree to which this exclusively reflects vascular inflammation in OSA is inconclusive. Adverse inflammatory profiles, as well as increased incidence of coronary events, have been associated with differential changes to EAT, in some cases culminating in decreased attenuation (22), and in others, increased attenuation (10,37). Furthermore, the anti-inflammatory effect of statin therapy has generated dissimilar results in previous studies on EAT: one study found a reduction in EAT attenuation independent of changes to lipid (38), while another reported no changes to EAT attenuation from statin use in a cohort of patients with subclinical CAD (39).
It is difficult, therefore, to attribute the phenomenon of decreased attenuation of EAT solely to inflammation and not other mechanisms operating within the vasculature. This is of particular importance to consider in light of our finding that patients with severe OSA and a high plaque burden had a significantly greater presence of calcified plaque, which may itself contribute to lower attenuation. Coronary artery calcification (CAC) typically reflects plaque stability rather than vulnerability and inflammation (40). CAC has been previously associated with decreased EAT attenuation (22,41,42), but this relationship was notably strongest after adjustment for coronary artery bypass surgery (42), which is associated with acute inflammation (43). This suggests that EAT attenuation undergoes conflicting changes under acute inflammatory conditions compared to the plaque-stabilising effects of calcification. Moreover, CAC disrupts the relationship between EAT volume and attenuation. Evidence from numerous observational studies indicates EAT attenuation decreases as EAT volume increases independent of statin use, cardiac surgery and cardiovascular risk factors (39,41,42). PCAT attenuation, likewise, decreases as EAT volume increases (33). However, Liu et al. found that as CAC increases, the association between EAT attenuation and volume becomes progressively diminished (10), suggesting calcification produces unique effects on EAT attenuation distinct from EAT volume. In conjunction with this, we found that decreased attenuation of EAT and PCAT was accompanied by only a numerical increase in the volume of these depots among patients with both severe OSA and high plaque burden. Collectively, these findings highlight the effect of calcification on EAT attenuation, and the potentially confounding role this may have played on the decreased attenuation of both EAT and PCAT observed in our study in patients with severe OSA and significant coronary plaque.
EAT attenuation in OSA independent of plaque burden
Accordingly, we adjusted for significant coronary calcification by solely studying a subgroup of patients with a low plaque burden and no differences in the prevalence of obesity or statin use. This revealed that patients with a low plaque burden and severe OSA harboured significantly higher EAT attenuation and numerically higher PCAT attenuation than those with only mild-moderate OSA. Poulain et al. (44) found that in non-obese mice chronic intermittent hypoxia resulted in numerous adipose tissue changes associated with an increased inflammatory state, including a localised increase in inflammatory cytokines and macrophages, and phenotypic changes to adipocytes such as decreased size and increased UCP-1 expression, which account for increased CT attenuation (11,45,46). Not only does this further highlight the inflammatory alterations occurring in adipose tissue after exposure to intermittent hypoxia, but that such changes occur independent of obesity and the accumulation of low attenuation adipose tissue. Moreover, a strong body of evidence from human studies highlights that vascular inflammation increases attenuation of PCAT, which is emerging as a robust marker of coronary risk proportional to the degree of atherosclerosis (47,48). Indeed, the changes adopted by pericoronary adipocytes as a result of exposure to vascular inflammatory mediators include decreased size and increased expression of inflammatory markers (11), and highlight that increased PCAT attenuation is a potent marker of an increased inflammatory microenvironment, such as that which would likely be induced in OSA. Despite PCAT attenuation being only numerically increased in our study, the finding of increased EAT attenuation highlights that OSA produces changes in EAT that are more consistent with vascular inflammation when the influence of CAD is minimised.
Therefore, unique compositional changes to EAT and PCAT can be captured by CT attenuation in patients with both severe OSA and significant coronary disease, presenting a novel means of quantifying coronary inflammatory risk among these patients in vivo. However, cardiometabolic diseases that often accompany OSA, such as obesity and CAD, can themselves cause distinct changes in adipose tissue. As we have found, interactions between these conditions may weaken associations between OSA and vascular inflammation, and produce heterogeneity in studies investigating adipose tissue characteristics in patients with OSA. Accordingly, future studies using CT attenuation as an imaging biomarker in this regard would require studying the intravascular implications of OSA in isolation from frequent comorbidities, so as to evaluate more precisely the extent of vascular inflammation effected by OSA alone. Indeed, Murphy et al. (14) reported that in humans without comorbidities, OSA severity was correlated with a marker of pro-inflammatory macrophage activity. Determining the role of vascular inflammation in OSA would enable potential stratification of inflammatory risk in these patients, and may warrant the implementation of anti-inflammatory agents in treatment strategies for OSA in addition to first-line CPAP therapy.
Limitations
Our study has a number of limitations. Firstly, the study cohort was derived from a single centre and from patients with a clinical indication for PSG, and as a result we were unable to derive a sufficiently large control group consisting of patients without OSA. Our study cohort was also small, as only patients with a sleep study within 12 months of coronary CTA were included. Furthermore, the retrospective study design and absence of follow-up data hampered investigation into potential temporal changes in adipose tissue on CT, particularly those that may have occurred as a result of treatments for OSA such as CPAP. Importantly, our capacity to determine the effect of severe OSA independent of the influence of coronary calcification was limited by the small sample size of patients with a low plaque burden.
Conclusions
OSA is associated with coronary plaque burden. The combination of severe OSA and high coronary plaque burden is associated with decreased EAT and PCAT attenuation. EAT attenuation is increased in patients with severe OSA but low plaque burden. The presence of divergent results in our study suggests a confounding effect between these two diseases. These findings are novel, but require validation by studies assessing inflammation in OSA patients after minimising the impact of coronary atherosclerosis and other comorbidities.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-338/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-338/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-338/coif). SJN received research support from Amgen, Anthera, AstraZeneca, Cerenis, Eli Lilly, Esperion, InfraReDx, Liposcience, Novartis, The Medicines Company, Resverlogix, Roche, Sanofi-Regeneron, The Medicines Company, and consulting fees from Akcea, Anthera, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Merck, Omthera, Resverlogix, Sanofi-Regeneron and Takeda. GSH received equipment to support research from ResMed, Philips Respironics and Air Liquide Healthcare. DW received honoraria for lectures from Eli-Lilly, Pfizer and Boehringer. DTLW serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from February 2021 to January 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Research Ethics Committee of Monash Health (No. EC00382) and informed consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mo L, Gupta V, Modi R, et al. Severe obstructive sleep apnea is associated with significant coronary artery plaque burden independent of traditional cardiovascular risk factors. Int J Cardiovasc Imaging 2020;36:347-55. [Crossref] [PubMed]
- Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005;112:2660-7. [Crossref] [PubMed]
- Mooe T, Rabben T, Wiklund U, et al. Sleep-disordered breathing in women: occurrence and association with coronary artery disease. Am J Med 1996;101:251-6. [Crossref] [PubMed]
- Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [Crossref] [PubMed]
- Kheirandish-Gozal L, Gozal D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int J Mol Sci 2019;20:459. [Crossref] [PubMed]
- Rosenquist KJ, Pedley A, Massaro JM, et al. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovasc Imaging 2013;6:762-71. [Crossref] [PubMed]
- Shibasaki I, Nishikimi T, Mochizuki Y, et al. Greater expression of inflammatory cytokines, adrenomedullin, and natriuretic peptide receptor-C in epicardial adipose tissue in coronary artery disease. Regul Pept 2010;165:210-7. [Crossref] [PubMed]
- Talman AH, Psaltis PJ, Cameron JD, et al. Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther 2014;4:416-29. [PubMed]
- Nerlekar N, Brown AJ, Muthalaly RG, et al. Association of Epicardial Adipose Tissue and High-Risk Plaque Characteristics: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2017;6:006379. [Crossref] [PubMed]
- Liu Z, Wang S, Wang Y, et al. Association of epicardial adipose tissue attenuation with coronary atherosclerosis in patients with a high risk of coronary artery disease. Atherosclerosis 2019;284:230-6. [Crossref] [PubMed]
- Antonopoulos AS, Sanna F, Sabharwal N, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 2017;9:eaal2658. [Crossref] [PubMed]
- Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018;392:929-39. [Crossref] [PubMed]
- He Q, Yang QC, Zhou Q, et al. Effects of varying degrees of intermittent hypoxia on proinflammatory cytokines and adipokines in rats and 3T3-L1 adipocytes. PLoS One 2014;9:e86326. [Crossref] [PubMed]
- Murphy AM, Thomas A, Crinion SJ, et al. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur Respir J 2017;49:1601731. [Crossref] [PubMed]
- Kaptoge S, Seshasai SR, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J 2014;35:578-89. [Crossref] [PubMed]
- Campbell AJ, Neill AM. Home set-up polysomnography in the assessment of suspected obstructive sleep apnea. J Sleep Res 2011;20:207-13. [Crossref] [PubMed]
- Chou KT, Chang YT, Chen YM, et al. The minimum period of polysomnography required to confirm a diagnosis of severe obstructive sleep apnoea. Respirology 2011;16:1096-102. [Crossref] [PubMed]
- Iber C, Ancoli-Israel S, Chesson AL, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine, 2007.
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597-619. [Crossref] [PubMed]
- de Araújo Gonçalves P, Garcia-Garcia HM, Dores H, et al. Coronary computed tomography angiography-adapted Leaman score as a tool to noninvasively quantify total coronary atherosclerotic burden. Int J Cardiovasc Imaging 2013;29:1575-84. [Crossref] [PubMed]
- Tran T, Small G, Cocker M, et al. A single slice measure of epicardial adipose tissue can serve as an indirect measure of total epicardial adipose tissue burden and is associated with obstructive coronary artery disease. Eur Heart J Cardiovasc Imaging 2014;15:423-30. [Crossref] [PubMed]
- Goeller M, Achenbach S, Marwan M, et al. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr 2018;12:67-73. [Crossref] [PubMed]
- Bikov A, Kolossváry M, Jermendy AL, et al. Comprehensive coronary plaque assessment in patients with obstructive sleep apnea. J Sleep Res 2019;28:e12828. [Crossref] [PubMed]
- Umut Somuncu M, Bulut U, Karakurt H, et al. The Relationship Between Obstructive Sleep Apnea and Coronary Plaque: A Coronary Computed Tomographic Angiography Study. Acta Cardiol Sin 2019;35:325-34. [PubMed]
- Wu J, Stefaniak J, Hafner C, et al. Intermittent Hypoxia Causes Inflammation and Injury to Human Adult Cardiac Myocytes. Anesth Analg 2016;122:373-80. [Crossref] [PubMed]
- Schiza SE, Mermigkis C, Panagiotis P, et al. C-reactive protein evolution in obstructive sleep apnoea patients under CPAP therapy. Eur J Clin Invest 2010;40:968-75. [Crossref] [PubMed]
- Panoutsopoulos A, Kallianos A, Kostopoulos K, et al. Effect of CPAP treatment on endothelial function and plasma CRP levels in patients with sleep apnea. Med Sci Monit 2012;18:CR747-51. [Crossref] [PubMed]
- Thunström E, Glantz H, Yucel-Lindberg T, et al. CPAP Does Not Reduce Inflammatory Biomarkers in Patients With Coronary Artery Disease and Nonsleepy Obstructive Sleep Apnea: A Randomized Controlled Trial. Sleep 2017; [Crossref] [PubMed]
- Mariani S, Fiore D, Barbaro G, et al. Association of epicardial fat thickness with the severity of obstructive sleep apnea in obese patients. Int J Cardiol 2013;167:2244-9. [Crossref] [PubMed]
- Kostopoulos K, Alhanatis E, Pampoukas K, et al. CPAP therapy induces favorable short-term changes in epicardial fat thickness and vascular and metabolic markers in apparently healthy subjects with obstructive sleep apnea-hypopnea syndrome (OSAHS). Sleep Breath 2016;20:483-93. [Crossref] [PubMed]
- Çetin S, Vural MG, Gündüz H, et al. Epicardial fat thickness regression with continuous positive airway pressure therapy in patients with obstructive sleep apnea: assessment by two-dimensional echocardiography. Wien Klin Wochenschr 2016;128:187-92. [Crossref] [PubMed]
- Andrews J, Nerlekar N, Gupta V, et al. Relationship Between Epicardial Fat Volume and Density with Obstructive Sleep Apnea and Coronary Plaque Burden. Circulation 2019;140:abstr A14097.
- Hell MM, Achenbach S, Schuhbaeck A, et al. CT-based analysis of pericoronary adipose tissue density: Relation to cardiovascular risk factors and epicardial adipose tissue volume. J Cardiovasc Comput Tomogr 2016;10:52-60. [Crossref] [PubMed]
- Skurk T, Alberti-Huber C, Herder C, et al. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 2007;92:1023-33. [Crossref] [PubMed]
- Henrichot E, Juge-Aubry CE, Pernin A, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol 2005;25:2594-9. [Crossref] [PubMed]
- Gozal D, Gileles-Hillel A, Cortese R, et al. Visceral White Adipose Tissue after Chronic Intermittent and Sustained Hypoxia in Mice. Am J Respir Cell Mol Biol 2017;56:477-87. [Crossref] [PubMed]
- Mahabadi AA, Balcer B, Dykun I, et al. Cardiac computed tomography-derived epicardial fat volume and attenuation independently distinguish patients with and without myocardial infarction. PLoS One 2017;12:e0183514. [Crossref] [PubMed]
- Raggi P, Gadiyaram V, Zhang C, et al. Statins Reduce Epicardial Adipose Tissue Attenuation Independent of Lipid Lowering: A Potential Pleiotropic Effect. J Am Heart Assoc 2019;8:e013104. [Crossref] [PubMed]
- Nerlekar N, Thakur U, Lin A, et al. The Natural history of Epicardial Adipose Tissue Volume and Attenuation: A long-term prospective cohort follow-up study. Sci Rep 2020;10:7109. [Crossref] [PubMed]
- Mori H, Torii S, Kutyna M, et al. Coronary Artery Calcification and its Progression: What Does it Really Mean? JACC Cardiovasc Imaging 2018;11:127-42. [Crossref] [PubMed]
- Abazid RM, Smettei OA, Kattea MO, et al. Relation Between Epicardial Fat and Subclinical Atherosclerosis in Asymptomatic Individuals. J Thorac Imaging 2017;32:378-82. [Crossref] [PubMed]
- Franssens BT, Nathoe HM, Visseren FL, et al. Relation of Epicardial Adipose Tissue Radiodensity to Coronary Artery Calcium on Cardiac Computed Tomography in Patients at High Risk for Cardiovascular Disease. Am J Cardiol 2017;119:1359-65. [Crossref] [PubMed]
- Kremen J, Dolinkova M, Krajickova J, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab 2006;91:4620-7. [Crossref] [PubMed]
- Poulain L, Thomas A, Rieusset J, et al. Visceral white fat remodelling contributes to intermittent hypoxia-induced atherogenesis. Eur Respir J 2014;43:513-22. [Crossref] [PubMed]
- Baba S, Jacene HA, Engles JM, et al. CT Hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. J Nucl Med 2010;51:246-50. [Crossref] [PubMed]
- Poulain L, Richard V, Lévy P, et al. Toll-like receptor-4 mediated inflammation is involved in the cardiometabolic alterations induced by intermittent hypoxia. Mediators Inflamm 2015;2015:620258. [Crossref] [PubMed]
- Lin A, Nerlekar N, Yuvaraj J, et al. Pericoronary adipose tissue computed tomography attenuation in different stages of coronary artery disease: a cross-sectional study. J Am Coll Cardiol 2020;75:1718. [Crossref]
- Goeller M, Tamarappoo BK, Kwan AC, et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2019;20:636-43. [Crossref] [PubMed]


