
Image-guided tunneled peritoneal dialysis catheter placement
Introduction
Patients with end-stage renal disease (ESRD) will ultimately require renal replacement therapy (dialysis or renal transplant) to survive (1). Renal transplantation is limited due to a global organ shortage, so most patients with ESRD are treated with dialysis (2). One type of dialysis is hemodialysis (HD), where blood is filtered externally to the body via an artificial membrane. The other is peritoneal dialysis (PD), where the vessel-rich peritoneum functions as a semipermeable membrane, expelling toxins and accumulated volume into a dialysate fluid within the peritoneal cavity via an osmotic interaction. The dialysate is introduced and drained through a long term tunneled catheter that exits through the skin in the abdomen (3,4). HD is typically performed three times weekly at an outpatient facility or rarely at home, while PD is performed at home. As a result, patients who utilize PD experience greater autonomy, higher satisfaction scores, and steadier volume status equilibrium, as there are shorter intervals between dialysis sessions (5). Patients are able to avoid outpatient centers entirely while still achieving adequate dialysis (6). PD is shown to achieve greater survival benefit one year after dialysis initiation compared to HD, although the mortality risk of these two approaches is equivalent at 5 years (7-10). The topic of HD versus PD has become increasingly relevant since the outbreak of COVID-19, as a tremendous shift has occurred in healthcare delivery favoring telemedicine and at-home healthcare in order to avoid the spread of infection in high risk individuals, which includes those with ESRD (11,12).
PD was first introduced approximately a century ago, however it was not relied upon solely for dialysis until 1959 (13). The first indwelling PD catheter was not developed until 1968, and this was inserted via an open surgical approach (14). Despite drastic improvements in the technology and delivery method of this dialysis approach, as well as cost effectiveness in countries that do not need to import the dialysate, PD remains less utilized than HD. In fact, in 2017, only 7.1% of ESRD patients utilized PD in the United States (15). Other countries such as Hong Kong and Thailand have increased the utilization of PD use through “Peritoneal Dialysis First” initiatives, as it is a more cost and resource conscious option if patients are functional enough to perform it themselves at home (16). In the US, many patients with ESRD have multiple comorbidities that result in an overall poor performance status, sometimes making HD a more attractive option, as dialysis sessions can occur in an outpatient facility where patients receive assistance from clinic staff and personnel rather than having to perform dialysis independently.
Despite its ease of home administration, PD has been measured to undergo technical failure at a rate of up to 0.35 episodes per patient year (17) and eventual transition to HD, as catheter failure, migration events, and infection can occur (3). In understanding different techniques that are utilized to place PD catheters, these complications may be minimized, and the efficacy and success of PD may be improved. The purpose of this review article is to discuss the various techniques that are utilized to place PD catheters, the rationale for patient selection, and advantages and disadvantages that are encountered with differing approaches.
Insertion methods
Interventional radiologists, surgeons, and nephrologists place tunneled PD catheters. Although tunneled PD catheters discussed in this article are placed via fluoroscopic (image-guided) techniques, primarily used by interventional radiologists, other methods exist. Surgeons place PD catheters via open surgical or laparoscopic approaches (18). Meta-analysis of these techniques compared to percutaneous fluoroscopic techniques demonstrate no significant difference between outcomes in catheter survival at 1-year, however surgical techniques can provide adjunct procedures such as omentopexy or omentectomy, limiting omental related complications which affect PD catheters, while fluoroscopic techniques offer the advantage of decreased invasiveness and accurate real-time imaging confirmation of catheter positioning throughout the procedure, with a lower overall rate of infectious and mechanical complications (19-21).
In some institutions, nephrologists place PD catheters, often using a non-fluoroscopic technique, using ultrasound guidance to achieve percutaneous access into the peritoneum and then advancing a guidewire and dilator to ultimately place the PD catheter in the abdomen (22). This approach has fallen out of favor as there is no direct visualization or imaging evidence that the catheter has been correctly placed in the dependent portion of the pelvis. Similar to interventional radiology, interventional nephrologists can use fluoroscopic guidance to confirm adequate positioning in the inferior deep pelvis (22). The first documented interventional radiology approach was described in 1992, and since then has evolved to involve fluoroscopy to aid accurate placement of the PD catheter in the cul de sac of the deep pelvis, known as the rectouterine space in females and the rectovesical space in males, the most gravity dependent portion of the peritoneum (23,24). While peritoneal dialysis was infrequently employed in its infancy, it has grown from being utilized in less than 8% of dialysis patients in 2008 to ~11% in 2018 in the United states, and ~21% of dialysis patients in Canada (25,26).
Regardless of approach, optimal positioning of the PD catheter in the abdomen is integral for achieving successful dialysis. This optimal positioning is intuitively achieved when the tip or coiled end of the PD catheter terminates in the most gravity dependent portion of the peritoneal cavity, allowing dialysate fluid to accumulate in this area, filter blood products through the peritoneal membrane, and empty through the catheter once the dialysate exchange is complete (27,28). This region is the rectovesical space in men and the rectouterine space in women, which is the most infero-posterior and gravity-dependent region where dialysate fluid will accumulate when a patient is supine during dialysis. Another advantage of having PD catheter in this region is that this area does not contain any tissue from the mesentery or greater omentum, limiting the risk of occluding catheter exit holes with abdominal tissue (29). Catheter migration out of this region is a common cause of peritoneal dialysis failure and is estimated to occur at a rate in as many as 12.7% to 35% of placed PD catheters (28,30,31). In many of these studies, these rates may even be underestimations, as catheter malposition was determined based on two-dimensional imaging, theoretically missing migrations parallel to the imaged plane-of-view, i.e., anterior migration events on an AP or PA plain radiograph.
Catheter types
PD catheters may be straight or contain a coiled terminus, and are made from medical grade silastic tubing. Coiled catheters are more frequently utilized for the purpose of PD in the United States due to the increased distal weight at the end of the catheter to minimize risk of migration, increased comfort of a rounded rather than a straight and blunt structure in the deep pelvis, as well as the added ease of locating the catheter on imaging studies due to its characteristic pigtail appearance (32,33). Dacron™ polyester felt cuffs are glued to the portion of the catheter that will reside in the rectus abdominus muscle as well as the superficial subcutaneous tissue track of the anterior abdominal wall, promoting adhesion and fibrous ingrowth of the catheter in these areas (Figure 1). In addition, the integration of Dacron™ cuffs around the catheter where it courses through soft tissue planes provides an added physical barrier to prevent infection, extrusion, and dialysis leak from the abdomen during dialysis (34). Catheter insertion site coincides with the deep cuff location, and is generally located by aligning the coiled portion of the PD catheter with the pubic symphysis and placing the remainder of the catheter superiorly, approximately 2–4 cm lateral of midline (35). The catheter courses through the subcutaneous tissues and can be tunneled at various degrees of length via connectors, and can exit the skin in the mid abdomen or as superiorly as the anterior chest depending on patient body habitus, panniculus, operator preference, and the type of catheter being used during the procedure. The parasternal anterior chest exit site is used less often in obese patients and those patients dependent on sitting in bathtubs for bathing, so that the exit site is not exposed to bath water. Most patients prefer an exit site within the upper abdomen, above the belt line. Some patients prefer the exit site in the low abdomen, below the belt line. To prepare the catheter, the PD catheter is flushed and soaked in normal saline without heparin to remove any free air in the catheter and cuffs that may interfere with fibrous ingrowth into the cuffs from the abdominal wall soft tissues after placement.

Patient selection and preparation
When selecting appropriate patients to undergo placement of a tunneled PD catheter for ESRD, very few absolute contraindications exist, particularly in the instance of percutaneous techniques, as the use of general anesthesia can be avoided entirely. In general, disease pathology involving the peritoneum is a direct contraindication both to have a tunneled peritoneal procedure performed, as well as being able to receive peritoneal dialysis in the future once a catheter is placed. Conditions that decrease efficacy of PD or serve as contraindications are active infection, colitis, diverticulitis, recent abdominal surgical instrumentation, and enterostomy. Relative contraindications are broad, and are frequently encountered, as patients who require peritoneal dialysis by definition have ESRD and tend to have multiple comorbidities associated with their renal failure including diabetes mellitus, obesity, polycystic kidney disease, heart failure, and history of abdominal surgery (36). However, in avoiding the use of general anesthesia, these comorbidities become less problematic, and the primary determinant involves a patient’s future ability to perform PD independently and effectively on a regular basis. Since PD is typically performed independently in a home environment, considerations such as home infrastructure, family support, visiting nursing staff, manual dexterity, overall mobility, and intact cognition are integral to successful PD.
Selection of catheter exit site in the anterior abdominal or chest wall is usually either above the beltline, below the beltline, or in the pre-sternal area of the chest, and this decision is made on a case-by-case basis with the patient (Figure 2). Then, the patient will undergo a bowel preparation regimen before the procedure (similar to a colonoscopy) to ensure the bowel is decompressed to minimize risk of inadvertent needle perforation of the bowel and provide greater room for catheter manipulation during PD catheter placement. Nothing by mouth status is recommended for six hours before the procedure since moderate sedation is used. Approximately one hour prior to the procedure, an intravenous prophylactic antibiotic such as cefazolin (or vancomycin if there is a history of MRSA infection) is administered. Finally, the urinary bladder should be empty to further minimize risk of iatrogenic perforation (37,38). The urinary bladder should be assessed with ultrasound prior to the procedure. If it is not empty, the patient should attempt to void, otherwise a single straight catheterization should be performed to empty the urinary bladder.
The patient is then placed supine, while the areas of potential access are shaven and prepped in a sterile manner using chlorhexidine gluconate or povidone-iodine.
Insertion technique
Conscious sedation is achieved with intravenous midazolam hydrochloride and fentanyl citrate, while vital signs are continuously monitored by nursing and physician staff members in the procedural suite. Some patients who cannot achieve adequate sedation with these medications or have other contraindications to conscious sedation will undergo general anesthesia with an anesthesiologist present during the entirety of the procedure.
Ultrasound guidance is utilized to localize a safe needle entry site into the anterior peritoneal cavity clear of bowel loops through the anterior abdominal wall, generally 2–4 cm lateral to midline in the abdomen at the level of the umbilicus. Using doppler waveforms, the location of the inferior epigastric artery is noted and avoided. Intradermal and subcutaneous 1% lidocaine is injected as a local anesthetic, and a 2–3 cm skin incision is made at the proposed puncture site. Initial percutaneous access is achieved using ultrasound guidance into the peritoneal cavity to visualize safe entry through subcutaneous soft tissues and the rectus abdominus muscle in the anterior abdominal wall, using a zig-zag abdominal wall puncture with a micropuncture needle or a Veress-type spring loaded needle (39). Percutaneous puncture using the chosen needle is made at a 45-degree angle towards the pubic symphysis, then flattened to travel through the rectus abdominus muscle for several centimeters, then again angled at 45 degrees downwards to travel into the peritoneal cavity, which is the zig-zag puncture. So that the distal catheter coil has room to course into the deep pelvis, the entry site should ideally be 15.5–16.5 cm from the rectovesical/rectouterine space. This distance range may vary depending on patient body habitus, height, and anatomic variants in the abdomen. Available catheter lengths are limited. Therefore, in large patients, the operator must choose an entry site as close as possible and in thin patients, far enough away to avoid intraperitoneal catheter redundancy. No practical technique exists for measuring this distance prior to the procedure, short of obtaining a CT scan of the pelvis, so an estimate is usually made.
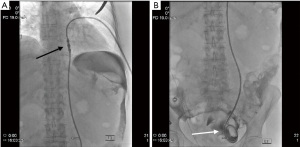
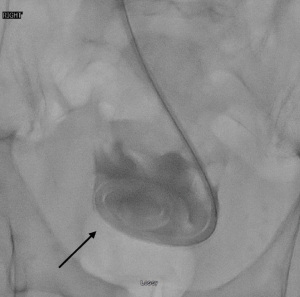
Once peritoneal cavity access is suspected, full strength iodinated contrast media is injected during fluoroscopic monitoring to perform a limited peritoneogram and assess the location of the access needle (Figure 3). This contrast media injection may also help reveal whether mesentery, bowel loop, or omentum may be interfering with adequate placement. If placement appears appropriate, 100–200 more mL of iodinated contrast media diluted with 100–200 mL of normal saline is then injected, resulting in a complete peritoneogram. The pelvic cul-de-sac should fill and become opacified on fluoroscopy in a “U-Shaped” pattern, as it is a pouch-shaped anatomical structure (29,40) (Figure 4). A straight Bentson 0.035-inch diameter guide wire is advanced into the peritoneum if using a Veress-type needle or a 0.018-inch diameter guide wire if using a micropuncture needle and the needle is removed. This guide wire is used to maintain access while an angled 4-5 French catheter is inserted over the guidewire into the peritoneum, and this first guidewire is exchanged for a 0.035-inch diameter regular or stiff hydrophilic guide wire such as the Glidewire™. The advantage of using this hydrophilic guide wire with an angled catheter is that these can be advanced in a posterior direction to move around bowel loops and mesenteric fat, eventually reaching the cul de sac which is filled with iodinated contrast media and well visualized fluoroscopically (Figure 5). Positioning confirmation may be achieved via manipulating the contrast media during real time fluoroscopy, the use of oblique or lateral projected radiographic imaging, or cone beam CT.
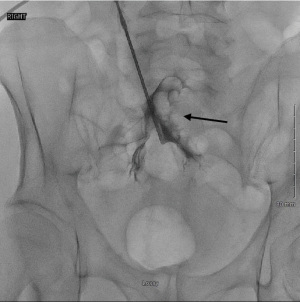

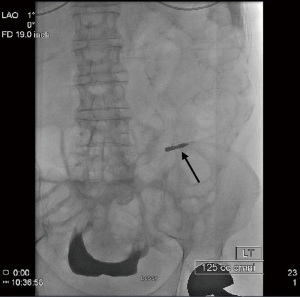
The hydrophilic guide wire is then replaced with a heavy-duty stiff 0.035-inch guide wire. Once this replacement is made, the 4–5 French Catheter may be removed, and the access track into the peritoneal cavity is dilated with serial dilators passed over the guidewire. This dilation allows placement of an 18 French peel-away sheath which will be placed into the posterior aspect of the deep pelvis, also known as the cul de sac. The introducer of the peel away sheath is then removed, but the stiff guide wire is left in place. The PD catheter can now be advanced over the stiff guide wire, through the peel away sheath, and into the cul de sac (Figure 6).
The patient’s skin exit site is then anesthetized intradermally with 1% lidocaine. The connection between this exit site and the periumbilical insertion site is made subcutaneously using either a plastic tunneling tool or a separate metallic tunneling trochar at a distance of approximately 4 cm (the distance between the two Dacron felt cuffs). This course should occur in a lateral and downward direction prior to exiting the body through the skin to limit contamination with skin flora, sweat, and other potential niduses of infection, which will promote drainage away from the novel subcutaneous track via gravity (37,41).
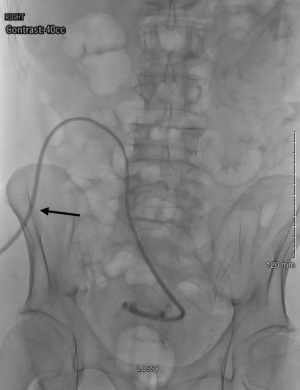
Once the skin exit site has been tunneled, the positioning of the catheter in the deep pelvis should be interrogated with imaging, such as the peritoneogram described above. Contrast media may be injected through the PD catheter during fluoroscopic recording to ensure patency of the catheter as well as confirming that the coil terminates in the cul de sac (Figure 7). If a kink or twist of the PD catheter or resistance is encountered with contrast media injection, advancing a guidewire through the catheter or a metallic repositioning stylet may be useful to help mitigate this obstruction. Assuming proper positioning of a patent catheter is achieved, a functional PD catheter test using 0.5 to 1.0 L of normal saline may be infused into the peritoneal space via gravity. If the bag is held 4 to 5 feet above the patient, this fluid is expected to infuse into the peritoneal cavity at a rate of greater than 100 mL/min. The drainage test may be performed with the bag of saline on the floor below the patient, and the normal saline should drain out of the peritoneal cavity at a rate of greater than 80 mL/min. The skin incision at the insertion site may be closed with tissue adhesive and Steri-strips, and generally does not need sutures.
With the procedure complete, a metallic luer lock adapter may be placed onto the end of the PD catheter, and an external extension catheter can be connected for ease of access. The entire catheter and extension are then covered with a watertight, sterile dressing such as Tegaderm, and the catheter track should heal for 5 to 10 days before any disruption of the dressing and interrogation of the PD catheter occurs. In general, PD is safe to initiate approximately three to four weeks after uncomplicated catheter placement (32).
Tunneled PD catheters may also be embedded with later extraction for delayed onset of PD, when a patient has some residual renal function and may not need to start PD for many months or years in the future (Figure 8). Management and troubleshooting recommendations of tunneled PD catheters is beyond the scope of this manuscript, but has been previously described (39).
Discussion
As the prevalence of ESRD in the United States continues to rise, so too does the pressure to perform procedures to achieve dialysis, regardless of whether HD or PD is utilized (15). However, a recent executive order in the United States has mandated that at least 80% of patients with ESRD receive either at home dialysis or a renal transplant in an effort to minimize the overall healthcare burden of ESRD on the US healthcare system (42). Also, governmental agencies are encouraging greater use of home dialysis and kidney transplants for Medicare beneficiaries with ESRD and are adjusting payments to ensure greater utilization of home dialysis rates (43). Since kidney transplant availability is limited and PD accounts for the majority of home dialysis, the burden of this will fall on clinicians to promote PD. On top of this, regional variations of COVID-19 incidence have resulted in variable access to healthcare at times of emergency and crisis, making at-home treatment even more desirable (44).
The use of home dialysis in ESRD patients has increased by nearly 93% in the past decade, although trends have shown greater relative increases in at home HD over PD (15). Persistent barriers still remain, as there are several requisites in functional status and cognitive capacity that are required to successfully achieve PD on a daily basis, and catheter migration still continues to occur regardless of catheter insertion technique (3,27,35). Despite these challenges, PD is usually more cost-effective compared to HD in developed nations (4,45).
Despite PD being cost effective in developed nations, resource-limited countries may encounter several cost-prohibitive barriers to delivering adequate PD to patients, as dialysate is less readily available, and trained surgeons may not be as ubiquitous as in the United States (45-47). An approach to decreasing cost would be to utilize percutaneous image guided methods instead of surgical techniques in placing and maintaining PD catheters after migration events, as this helps avoid the use of general anesthesia and invasive and costly surgery, while preserving equivalent PD catheter survival rates. It has been estimated that 1- and 2-year survival rates for image guided percutaneous placed catheters range from 65–90% and 49–82% respectively. In contrast, surgically placed PD catheters possess a 1- and 2-year survival rate of 62–73% and 41–60% respectively (48-51). This may be beneficial in developed nations as well, as PD tunneled catheter procedures may be performed at an overall expedient rate and lower incidental hospital cost compared to open surgical and laparoscopic techniques (52). All of the insertion techniques have the ability to place a purse-string suture around the deep cuff of the PD catheter within the rectus abdominus muscle, decreasing the risk of dialysate leak and migration within anterior abdominal wall soft tissues, allowing for “early start” PD.
The possible complications of percutaneous techniques overlap heavily with surgical techniques, the most common of which is infection, has estimated to occur in up to 40% of patients who undergo percutaneous procedures in long term meta-analysis within one year, compared to studies which demonstrate infection rates up to 57% using open surgical approach. Mechanical complications are estimated to occur in up to 30% of percutaneously placed catheters, however meta-analyses have shown that surgical techniques again have a complication rate of approximately 36%. These results may be in part confounded by patient selection factors, as patients with more comorbidities, history of abdominal surgery, and morbid obesity tended to be selected for surgical approach over percutaneous technique (21). Occurring at a lower frequency, hematoma formation, unintended catheter leak, bleeding, bowel perforation, and iatrogenic hernia formation have also been reported to occur (21,53,54).
Laparoscopic surgical techniques have demonstrated lower rates of mechanical PD catheter failure compared to image-guided percutaneous techniques in some instances, specifically when omentopexy and adhesion lysis is performed, which cannot be accomplished during image-guided percutaneous insertion (19,20,55). However, these cases represent a small proportion of patients compared to the anticipated rise of home dialysis, which intuitively will necessitate more tunneled PD catheter placements. At institutions that rely solely on laparoscopic surgery insertion techniques, barriers to patients with ESRD may become related to scheduling timely PD catheter placement in the operating room, as well as increased scheduling difficulties encountered with general anesthesia services. If more PD catheters are initially placed using image guided percutaneous techniques, laparoscopic approaches may become more available for scheduling complicated patients requiring omentopexy, omentectomy, and adhesion lysis, while less complex patients can avoid surgical intervention entirely. Patients with a previous history of abdominal or pelvic surgery with adhesions or obese patients with a large amount of omental fat may be better candidates for laparoscopic placement.
Utilizing image-guided percutaneous techniques more frequently for tunneled PD catheter placement also provides a more instantaneous solution to mechanical catheter failure events, as many of these events may be attributed to kinking and migration that can be solved with simple guidewire manipulation, and adequate catheter positioning can be confirmed with imaging without ever converting to more invasive techniques (24,31,37). While some patients may eventually require laparoscopic or open repair for PD catheter failure, the minimal invasiveness that the image-guided percutaneous technique offers makes it an attractive option for a first attempt at repair rather than immediately escalating to surgical techniques and adds the benefit of being able to triage complexity of catheter problems prior to surgical consultation.
COVID-19 considerations
The COVID-19 pandemic has put a tremendous strain on healthcare workers and patients alike. Demand for medical care worldwide has increased both providers’ and patients’ risk for infection in the hospital environment. Preventing COVID-19 infection in patients receiving dialysis is particularly important because of the significant morbidity and mortality from infectious diseases associated with ESRD (56). While social distancing and increased mask utilization has been useful in preventing the spread of COVID-19, it is not always practical for healthcare providers to care for dialysis patients from six feet away in a hospital setting. For example, dialysis nurses must be in close contact with several patients during the cannulation process for HD, which increases transmission risk (57). Furthermore, if dialysis patients become exposed to COVID-19, then they must be triaged and quarantined accordingly. This would mean dedicated machines, dialysate, and spaces for this set of patients. This may lead to a further shortage of supplies and increases demand on dialysis nurses, technicians, and other healthcare providers during a pandemic that has already demonstrated a mismatch between supply and demand of medical personnel and supplies. These theoretical barriers may be avoided entirely by maximizing usage of at-home peritoneal dialysis.
In addition to the medical supply shortages and logistical difficulties that the COVID-19 pandemic has generated, psychological burden on patients has been a major problem as well. Traveling to and from dialysis centers three times a week, knowing that every dialysis session puts that individual at risk for contracting COVID-19 undoubtedly has taken a psychological toll on HD patients. In fact, when surveyed using the Impact of Event Scale (IES), HD patients reported a significantly higher IES score than PD patients despite receiving more psychological and in-person support from medical personnel (58). Some of these psychological stressors could be alleviated if dialysis were completed at home, avoiding multiple weekly trips to dialysis centers that put patients at risk for COVID-19 exposure.
The COVID-19 pandemic has forced the medical world to reevaluate the way healthcare is delivered and to refine existing models of delivery. The popularity of center-based HD versus at-home PD continues to be questioned, now with particular emphasis on preventing COVID-19 exposure in patients on dialysis. In the context of the COVID-19 pandemic, ESRD patients are extremely vulnerable and have a high risk of hospitalization at baseline. A transition to PD in appropriate candidate patients may significantly reduce these possible risks. In the United Kingdom in 2020, only 2.9% of patients on PD tested COVID-19 positive, while 9% of patients who received center-based HD contracted COVID-19 (59). Transitioning, however, will take some time, as PD requires training, higher functional status, and home equipment (60). Between COVID-19-related mortality and improving patient education, PD will hopefully become a progressively more attractive option.
Conclusions
Interventional radiology is a viable yet infrequently utilized resource to place and maintain tunneled PD catheters. The added benefit of minimal invasiveness, avoidance of general anesthesia in most cases, and real-time imaging confirmation of PD catheter placement provides unique advantages at a time when home dialysis is becoming increasingly relevant in patients with ESRD (37). With the large paradigm shift occurring in healthcare delivery from COVID-19, at home dialysis delivery will inevitably become integral in minimizing outpatient appointments, long wait times, and unnecessary exposures to infection in high-risk patients (12). PD remains the most common form of at-home dialysis compared to HD. An increasing demand will be met with increased need for image-guided percutaneous placement of tunneled PD catheters (15). This technique offers a cost-effective approach with low rates of peri-procedural complication, and can be scheduled expediently on an outpatient basis at many institutions, further decreasing costs associated with inpatient hospitalization (48-52). While surgical techniques remain valuable in troubleshooting complex patients requiring omentopexy, omentectomy, and lysis of adhesions, image-guided percutaneous tunneled PD catheter placement is becoming increasingly available to accommodate a wide variety of patients with ESRD while still achieving equivalent long-term PD catheter survival rates as those placed by surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sasan Partovi and Lee Kirksey) for the series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-579/coif). The series “Endovascular and Surgical Interventions in the End Stage Renal Disease Population” was commissioned by the editorial office without any funding or sponsorship. GJ reports UVMMC Radiology funding received for travel for poster presentation at ECTRIMS conference in 2019, and will be funding his travel for RSNA in Chicago in 11/2021. CM reports Patent pending: Peritoneal dialysis catheter weighted anchor modification. This is a modification of existing peritoneal dialysis catheters to increase efficacy and decrease complications. No payment, external grant or funding, royalty, consulting fee, or any financial interest has occurred related to this activity in the past 36 months. Christopher Morris did receive an internal grant to fund a study related to this medical device. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pastan S, Bailey J. Dialysis therapy. N Engl J Med 1998;338:1428-37. [Crossref] [PubMed]
- Bastani B. The present and future of transplant organ shortage: some potential remedies. J Nephrol 2020;33:277-88. [Crossref] [PubMed]
- Sinnakirouchenan R, Holley JL. Peritoneal dialysis versus hemodialysis: risks, benefits, and access issues. Adv Chronic Kidney Dis 2011;18:428-32. [Crossref] [PubMed]
- Berger A, Edelsberg J, Inglese GW, et al. Cost comparison of peritoneal dialysis versus hemodialysis in end-stage renal disease. Am J Manag Care 2009;15:509-18. [PubMed]
- Juergensen E, Wuerth D, Finkelstein SH, et al. Hemodialysis and Peritoneal Dialysis: Patients’ Assessment of Their Satisfaction with Therapy and the Impact of the Therapy on Their Lives. Clin J Am Soc Nephrol 2006;1:1191-6. [Crossref] [PubMed]
- Rubin HR, Fink NE, Plantinga LC, et al. Patient ratings of dialysis care with peritoneal dialysis vs hemodialysis. JAMA 2004;291:697-703. [Crossref] [PubMed]
- Tokgoz B. Clinical advantages of peritoneal dialysis. Perit Dial Int 2009;29:S59-61. [Crossref] [PubMed]
- Hansson JH, Watnick S. Update on Peritoneal Dialysis: Core Curriculum 2016. Am J Kidney Dis 2016;67:151-64. [Crossref] [PubMed]
- Mehrotra R, Devuyst O, Davies SJ, et al. The Current State of Peritoneal Dialysis. J Am Soc Nephrol 2016;27:3238-52. [Crossref] [PubMed]
- Weinhandl ED, Foley RN, Gilbertson DT, et al. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 2010;21:499-506. [Crossref] [PubMed]
- Parapiboon W, Ponce D, Cullis B. Acute peritoneal dialysis in COVID-19. Perit Dial Int 2020;40:359-62. [Crossref] [PubMed]
- Ronco C, Manani SM, Giuliani A, et al. Remote patient management of peritoneal dialysis during COVID-19 pandemic. Perit Dial Int 2020;40:363-7. [Crossref] [PubMed]
- Negoi D, Khanna R. History of Peritoneal Dialysis. In: Khanna R, Krediet RT, (eds). Nolph and Gokal's Textbook of Peritoneal Dialysis. Springer, Cham 2021;1-26.
- Tenckhoff H, Curtis FK. Experience with maintenance peritoneal dialysis in the home. Trans Am Soc Artif Intern Organs 1970;16:90-5. [PubMed]
- Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2020;75:A6-7. [Crossref] [PubMed]
- Liu FX, Gao X, Inglese G, et al. A Global Overview of the Impact of Peritoneal Dialysis First or Favored Policies: An Opinion. Perit Dial Int 2015;35:406-20. [Crossref] [PubMed]
- Htay H, Cho Y, Pascoe EM, et al. Multicenter Registry Analysis of Center Characteristics Associated with Technique Failure in Patients on Incident Peritoneal Dialysis. Clin J Am Soc Nephrol 2017;12:1090-9. [Crossref] [PubMed]
- Sun ML, Zhang Y, Wang B, et al. Randomized controlled trials for comparison of laparoscopic versus conventional open catheter placement in peritoneal dialysis patients: a meta-analysis. BMC Nephrol 2020;21:60. [Crossref] [PubMed]
- Crabtree JH, Fishman A. Selective performance of prophylactic omentopexy during laparoscopic implantation of peritoneal dialysis catheters. Surg Laparosc Endosc Percutan Tech 2003;13:180-4. [Crossref] [PubMed]
- Goh YH. Omental folding: a novel laparoscopic technique for salvaging peritoneal dialysis catheters. Perit Dial Int 2008;28:626-31. [Crossref] [PubMed]
- Tullavardhana T, Akranurakkul P, Ungkitphaiboon W, et al. Surgical versus percutaneous techniques for peritoneal dialysis catheter placement: A meta-analysis of the outcomes. Ann Med Surg (Lond) 2016;10:11-8. [Crossref] [PubMed]
- Asif A. Peritoneal dialysis catheter insertion. Minerva Chir 2005;60:417-28. [PubMed]
- Jacobs IG, Gray RR, Elliott DS, et al. Radiologic placement of peritoneal dialysis catheters: preliminary experience. Radiology 1992;182:251-5. [Crossref] [PubMed]
- Savader SJ. Percutaneous radiologic placement of peritoneal dialysis catheters. J Vasc Interv Radiol 1999;10:249-56. [Crossref] [PubMed]
- Klomjit N, Kattah AG, Cheungpasitporn W. The Cost-effectiveness of Peritoneal Dialysis Is Superior to Hemodialysis: Updated Evidence From a More Precise Model. Kidney Med 2020;3:15-7. [Crossref] [PubMed]
- Burkart J. The future of peritoneal dialysis in the United States: optimizing its use. Clin J Am Soc Nephrol 2009;4:S125-31. [Crossref] [PubMed]
- Gokal R, Alexander S, Ash S, et al. 1998 Update: (Official Report from the International Society for Peritoneal Dialysis). Perit Dial Int 1998;18:11-33. [Crossref] [PubMed]
- Eklund BH. Surgical implantation of CAPD catheters: presentation of midline incision-lateral placement method and a review of 110 procedures. Nephrol Dial Transplant 1995;10:386-90. [PubMed]
- Bordoni B, Sugumar K, Leslie SW. Anatomy, Abdomen and Pelvis, Pelvic Floor. 2021 Jul 21. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
- Bay WH, Cerilli GJ, Perrine V, et al. Analysis of a new technique to stabilize the chronic peritoneal dialysis catheter. Am J Kidney Dis 1983;3:133-5. [Crossref] [PubMed]
- Chen WM, Cheng CL. A simple method to prevent peritoneal dialysis catheter tip migration. Perit Dial Int 2007;27:554-6. [Crossref] [PubMed]
- Crabtree JH, Shrestha BM, Chow KM, et al. ISPD GUIDELINES/RECOMMENDATIONS. Perit Dial Int 2019;39:414-36. [Crossref] [PubMed]
- Gadallah MF, Mignone J, Torres C, et al. The role of peritoneal dialysis catheter configuration in preventing catheter tip migration. Adv Perit Dial 2000;16:47-50. [PubMed]
- Eklund B, Honkanen E, Kyllönen L, et al. Peritoneal dialysis access: prospective randomized comparison of single-cuff and double-cuff straight Tenckhoff catheters. Nephrol Dial Transplant 1997;12:2664-6. [Crossref] [PubMed]
- Peppelenbosch A, van Kuijk WH, Bouvy ND, et al. Peritoneal dialysis catheter placement technique and complications. NDT Plus 2008;1:iv23-8. [PubMed]
- Eroglu E, Heimbürger O, Lindholm B. Peritoneal dialysis patient selection from a comorbidity perspective. Semin Dial 2020; [Epub ahead of print]. [PubMed]
- Abdel-Aal AK, Dybbro P, Hathaway P, et al. Best practices consensus protocol for peritoneal dialysis catheter placement by interventional radiologists. Peritoneal Dialysis International 2014;34:481-93. [Crossref] [PubMed]
- Szeto CC, Li PK, Johnson DW, et al. ISPD Catheter-Related Infection Recommendations: 2017 Update. Perit Dial Int 2017;37:141-54. [Crossref] [PubMed]
- Morris CS. Interventional Radiology Placement and Management of Tunneled Peritoneal Dialysis Catheters: A Pictorial Review. Radiographics 2020;40:1789-806. [Crossref] [PubMed]
- Kahai P, Mandiga P, Wehrle CJ, Lobo S. Anatomy, Abdomen and Pelvis, Large Intestine. 2021 Aug 11. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
- Crabtree JH, Burchette RJ. Prospective comparison of downward and lateral peritoneal dialysis catheter tunnel-tract and exit-site directions. Perit Dial Int 2006;26:677-83. [Crossref] [PubMed]
- Trump Executive Order To Encourage In-Home Dialysis And More Kidney Donations : Shots - Health News : NPR. Available online: https://www.npr.org/sections/health-shots/2019/07/10/740276389/trump-administration-announces-plans-to-shake-up-the-kidney-care-industry (accessed 15 August 2021).
- HHS To Transform Care Delivery for Patients with Chronic Kidney Disease | CMS. Available online: https://www.cms.gov/newsroom/press-releases/hhs-transform-care-delivery-patients-chronic-kidney-disease (accessed 29 August 2021).
- Sun F, Matthews SA, Yang TC, et al. A spatial analysis of the COVID-19 period prevalence in U.S. counties through June 28, 2020: where geography matters? Ann Epidemiol 2020;52:54-59.e1. [Crossref] [PubMed]
- Pike E, Hamidi V, Ringerike T, et al. More Use of Peritoneal Dialysis Gives Significant Savings: A Systematic Review and Health Economic Decision Model. J Clin Med Res 2017;9:104-16. [Crossref] [PubMed]
- van de Luijtgaarden MW, Jager KJ, Stel VS, et al. Global differences in dialysis modality mix: the role of patient characteristics, macroeconomics and renal service indicators. Nephrol Dial Transplant 2013;28:1264-75. [Crossref] [PubMed]
- Karopadi AN, Mason G, Rettore E, et al. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant 2013;28:2553-69. [Crossref] [PubMed]
- Georgiades CS, Geschwind JF. Percutaneous peritoneal dialysis catheter placement for the management of end-stage renal disease: technique and comparison with the surgical approach. Tech Vasc Interv Radiol 2002;5:103-7. [Crossref] [PubMed]
- Ozener C, Bihorac A, Akoglu E. Technical survival of CAPD catheters: comparison between percutaneous and conventional surgical placement techniques. Nephrol Dial Transplant 2001;16:1893-9. [Crossref] [PubMed]
- Gadallah MF, Pervez A, el-Shahawy MA, et al. Peritoneoscopic versus surgical placement of peritoneal dialysis catheters: a prospective randomized study on outcome. Am J Kidney Dis 1999;33:118-22. [Crossref] [PubMed]
- Allon M, Soucie JM, Macon EJ. Complications with permanent peritoneal dialysis catheters: experience with 154 percutaneously placed catheters. Nephron 1988;48:8-11. [Crossref] [PubMed]
- Voss D, Hawkins S, Poole G, et al. Radiological versus surgical implantation of first catheter for peritoneal dialysis: a randomized non-inferiority trial. Nephrol Dial Transplant 2012;27:4196-204. [Crossref] [PubMed]
- Abdel Aal AK, Guest SS, Moawad S, et al. Outcomes of fluoroscopic and ultrasound-guided placement versus laparoscopic placement of peritoneal dialysis catheters. Clin Kidney J 2018;11:549-54. [Crossref] [PubMed]
- Henderson S, Brown E, Levy J. Safety and efficacy of percutaneous insertion of peritoneal dialysis catheters under sedation and local anaesthetic. Nephrol Dial Transplant 2009;24:3499-504. [Crossref] [PubMed]
- Krezalek MA, Bonamici N, Lapin B, et al. Laparoscopic peritoneal dialysis catheter insertion using rectus sheath tunnel and selective omentopexy significantly reduces catheter dysfunction and increases peritoneal dialysis longevity. Surgery 2016;160:924-35. [Crossref] [PubMed]
- Seidel M, Hölzer B, Appel H, et al. Impact of renal disease and comorbidities on mortality in hemodialysis patients with COVID-19: a multicenter experience from Germany. J Nephrol 2020;33:871-4. [Crossref] [PubMed]
- Chen TH, Wen YH, Chen CF, et al. The advantages of peritoneal dialysis over hemodialysis during the COVID‐19 pandemic. Semin Dial 2020;33:369-71. [Crossref] [PubMed]
- Xia X, Wu X, Zhou X, et al. Comparison of Psychological Distress and Demand Induced by COVID-19 during the Lockdown Period in Patients Undergoing Peritoneal Dialysis and Hemodialysis: A Cross-Section Study in a Tertiary Hospital. Blood Purif 2021;50:319-27. [Crossref] [PubMed]
- Brown EA, Perl J. Increasing Peritoneal Dialysis Use in Response to the COVID-19 Pandemic: Will It Go Viral? J Am Soc Nephrol 2020;31:1928-30. [Crossref] [PubMed]
- Stern LD, Waikar S. Time to Expand Access and Utilization of Home Dialysis: Lessons From the COVID-19 Pandemic. Mayo Clin Proc 2020;95:1323-4. [Crossref] [PubMed]



