Efficacy and safety of vorapaxar for the prevention of adverse cardiac events in patients with coronary artery disease: a meta-analysis
Introduction
As a consequence of the rapid modernization of the developing world, coronary artery disease (CAD) has become a leading cause of morbidity and mortality worldwide with a prevalence that reaches pandemic levels (1). The thrombosis caused by platelet activation in CAD patients may lead to the ischemia cardiovascular and cerebrovascular events. The standard dual anti-platelet therapy (aspirin combined with thienopyridine) can effectively decrease the incidences of these adverse clinical events and is widely used as a secondary prevention strategy in CAD patients (2). However, quite a number of patients who have already received standard dual anti-platelet therapy even still suffer from recurrent ischemic cardiovascular and cerebrovascular events. To further reduce these adverse events, novel anti-platelet agents are needed (3).
Protease-activated receptor-1 (PAR-1) is an important receptor which mediates the activation of platelet in a different pathway with thromboxane A2 and P2Y12 receptor. It plays a non-ignorable role in the process of platelet activation under the standard dual anti-platelet therapy (4). Vorapaxar is the first PAR-1 antagonist approved by the U.S. Food and Drug Administration for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction (MI) and peripheral artery disease, without a previous stroke or transient ischemic attack (5). In recent years, several randomized controlled trials (RCTs) have been conducted to evaluate the efficacy of vorapaxar in the treatment of CAD. However, the results were not consistent (6). Some studies found that vorapaxar was helpful in reducing the ischemic risk in patients with atherothrombotic processes (6). However, several other studies did not show any efficacy outcomes but an increasing risk of bleeding events (7-9). Therefore, the efficacy and safety of vorapaxar in reducing the incidence of adverse cardiovascular and cerebrovascular events should be systematically evaluated. Here in this study, we performed a meta-analysis of eligible studies to assess the efficacy and safety of vorapaxar in CAD patients.
Methods
Study search strategy
Relevant studies were identified from PubMed, Scopus, Cochrane Library and Embase using the following terms: vorapaxar, CAD, coronary heart disease and SCH530348. The retrieval time restricted from January 1st, 2008 to November 1st, 2015. Articles were limited to English-language studies. The included participants were patients with CAD who did not receive urgent percutaneous coronary intervention (PCI). If the same patient population was included in other studies, only the most recent study was considered in this meta-analysis.
Inclusion criteria
Eligible studies should meet all the following criteria: (I) only RCTs were considered; (II) participants should be patients with CAD and did not receive urgent PCI; (III) comparison should be made between two groups: vorapaxar group (vorapaxar + dual anti-platelet therapy) and placebo group (placebo + dual anti-platelet therapy); (IV) articles should report the efficacy and safety outcomes of vorapaxar. Meeting abstracts, case reports, editorials and reviews were excluded.
Data extraction and assessment
Two investigators independently extracted data from the included studies. Data extraction included the first author’s surname, publication year, region, demographic data, target population, treatment protocol, follow-up period, efficacy outcomes and safety outcomes. Disagreement was dissolved by discussion between the two investigators.
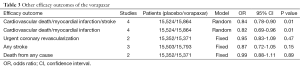
The primary efficacy outcome in this study was major adverse cardiovascular events (MACE) which was defined as a composite of cardiovascular death, MI, stroke, urgent coronary revascularization and recurrent ischemia with rehospitalization. The primary safety outcomes included thrombolysis in myocardial infarction (TIMI) major or minor bleeding and Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) severe or moderate bleeding. The definition of bleeding events was shown in Table 1.

Full table
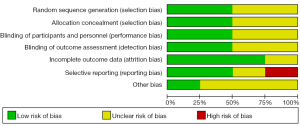
The quality of the studies was evaluated with the Jadad assessment scale. The grading is mainly based on the generation of the randomization, the application of the double-blind method and the instruction of the loss to follow-up (10). The full mark is 5 points. If the article is marked as 3 points or higher, it would be considered to be of high quality. The Cochrane Collaboration’s tool was also used to assess the risk of bias about the included studies. Random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other potential threats to validity are the judge entries. These two scales have revealed that the studies included here are qualified.
Statistical analysis
Meta-analysis review methodology was used for this study. Odds ratio (OR) with 95% confidence interval (CI) was used. P value ≤0.10 was considered to be significant for statistical heterogeneity. If P value ≤0.10, a random-effect model was chosen. Otherwise, a fixed-effect model was used instead. The pooled OR was performed for dominant model. All statistical tests were performed with RevMan 5.2.
Results
Eligible studies
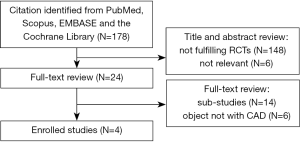
A total of 178 potentially eligible studies were identified, and 148 were excluded for not fulfilling inclusion criteria on the basic of RCTs. Twenty-six studies were excluded for other reasons, such as different studies but with the same population. A flow diagram of the trial selection process was given in Figure 1. Funnel plot was shown in Figure 2. The Cochrane Collaboration’s tool was also used (Figure 3).
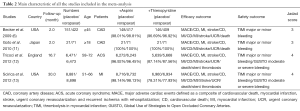
After the screening, four RCTs (6,11-13) incorporating 31,388 patients met the inclusion criteria and were included in this meta-analysis (Table 2). Of the four studies, sample size ranged from 92 to 17, 779 and the study period ranged from 2 to 30 months. No significant difference had been observed in the gender and age distribution between the vorapaxar and placebo group in each study.
Full table
Efficacy
Major adverse cardiovascular events (MACE)
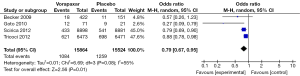
Four studies (6,11-13) incorporating 15,864 patients in vorapaxar group and 15,524 patients in placebo group were included in this analysis. The incidence of MACE was 12.5% (1,983/15,864) in the vorapaxar group while that was 14.0% (2,175/15,524) in the placebo group. There was a significant decrease in the incidence of MACE when adding vorapaxar to the standard dual anti-platelet therapy (OR, 0.86, 95% CI: 0.75–0.99, P=0.03) (Figure 4).

Myocardial infarction (MI)
Four studies (6,11-13) were included in this analysis. The incidence of MI in the vorapaxar group was 6.83% while that was 8.11% in the placebo group. Patients who received the vorapaxar had a lower risk of MI than those received placebo (OR, 0.79, 95% CI: 0.67–0.95, P=0.01) (Figure 5).
Cardiovascular death
Four studies (6,11-13) incorporating 15,864 in vorapaxar group and 15,524 in placebo group were included in this analysis. The incidence of cardiovascular death in the vorapaxar group was 2.26% while that was 2.43% in the placebo group. There was no significant difference among the two groups in the occurrence of cardiovascular death (OR, 0.95, 95% CI: 0.82–1.09, P=0.45) (Figure 6).

Ischemic stroke
Two studies (12,13) incorporating 15,371 patients taking vorapaxar and 15,352 patients taking placebo were included in this analysis. The incidence of ischemic stroke in the vorapaxar group was 0.93% while that was 1.29% in the placebo group. The vorapaxar group showed a lower risk in ischemic stroke than the placebo group (OR, 0.72, 95% CI: 0.58–0.89, P=0.003) (Figure 7).

Other efficacy outcomes
The risk of some other cardiovascular and cerebrovascular events between the two groups had also been assessed. The result had been shown in Table 3. Some positive outcomes had been observed. It indicated that vorapaxar combined with the dual-antiplatelet therapy could lower the risk of cardiovascular death or MI or stroke (OR, 0.84, 95% CI: 0.78-0.90, P=0.01) (Table 3) and cardiovascular death or MI (OR, 0.82, 95% CI: 0.69–0.96, P=0.01) (Table 3).
Full table
Safety
TIMI major or minor bleeding
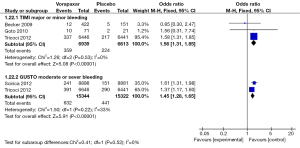
Three studies (6,11,12) incorporating 6,939 in the vorapaxar group and 6,613 in the placebo group were included in the TIMI major or minor bleeding assessment. Patients who received vorapaxar got a bleeding rate of 5.17% while it was 3.39% in those who received placebo. It indicated that patients receiving vorapaxar had a higher risk in TIMI major or minor bleeding (OR, 1.56, 95% CI: 1.31–1.85, P<0.00001) (Figure 8).
GUSTO moderate and severe bleeding
Another analysis about bleeding assessment was GUSTO moderate and severe bleeding. Two studies (12,13) incorporating 15,344 patients in vorapaxar group and 15,322 patients in placebo group were included in GUSTO moderate and severe bleeding assessment. Similarly, it showed that patients who received vorapaxar had a higher risk in GUSTO moderate and severe bleeding with a bleeding rate of 4.1% in contrast to 2.88% (OR, 1.45, 95% CI: 1.28–1.65, P<0.00001) (Figure 8).
Discussion
The present meta-analysis is consisted of four qualified RCTs (6,11-13) with 31,388 patients enrolled. The vorapaxar group could significantly lower the incidence of MACE, MI and ischemia stroke than the placebo group. But no significant difference of cardiovascular death was observed between the two groups. On the other hand, adding vorapaxar to the standard dual anti-platelet therapy increased the risk of bleeding, including TIMI major or minor bleeding events and GUSTO moderate and severe bleeding events.
In the standard dual anti-platelet therapy of patients with CAD, aspirin and thienopyridine are recommended to be used in combination (14-16). Aspirin is an irreversible inhibitor of cyclooxygenase-1 that produces a permanent defect in thromboxane A2-mediated platelet activation (17), while thienopyridine prevents adenosine diphosphate-induced platelet activation and aggregation by irreversibly inhibiting the P2Y12 receptor (17,18). The combining use of these two drugs can effectively reduce thrombosis. However, 12-month risk of recurrent vascular events remains at approximately 10% among CAD patients (19). It suggests that some other mechanisms may also play an important role, such as the thrombin-mediated pathway (20).
Vorapaxar, a novel oral anti-platelet agent, is a competitive, reversible antagonist of PAR-1, which can block thrombin-induced platelet activation. In some phase II studies, vorapaxar had been proved to be a key contributor in the platelet activation, while play a relatively secondary role in hemostasis (21,22). But there was still some phase III (12,23) studies showed that the combining use of three anti-platelet agents (aspirin, clopidogrel and vorapaxar) increased the risk of intracranial hemorrhage. In the past few years, a series of phase II and phase III studies (6,8,24,25) about vorapaxar had been conducted in different countries, but no consensus conclusion had been made on its efficacy and safety outcomes. Capodanno and his colleagues (26) summarized the relative literatures and conducted a meta-analysis in 2012, which showed that the PAR-1 antagonist may decrease ischemic events in patients with CAD as compared with placebo at the cost of an increased risk of clinically significant bleeding. However, the population enrolled in the meta-analysis was not the same on the basic disease, which might have an impact on the result of the study. In order to comprehensively evaluate the effect of vorapaxar on patients with CAD, we conduct the present updated meta-analysis.
In this pooled analysis, the population in the four included studies (6,11-13) was restricting to the patients with CAD who did not need an urgent PCI, which reduced the confounder caused by the different basic disease. The results concluded from this analysis were similar with some of the studies, indicating that vorapaxar could significantly lower the incidence of MACE (11,13), MI (11-13) and ischemia stroke than the placebo group (13). However, adding vorapaxar to the standard dual anti-platelet therapy did not lower the risk of cardiovascular death and may increase the risk of bleeding events. In this case, clinicians should weigh the pros and cons.
Some limitations should be taken into consideration. Firstly, only four qualified studies were enrolled in this meta-analysis, and two (6,11) of which got small sample sizes as well as rather short follow-up periods. Moreover, no fatal bleeding or intracranial haemorrhage events were evaluated in these two studies. Secondly, because of the limitation of the language, only journals published in Chinese and English have been reviewed. And there was no RCT about vorapaxar published in Chinese literatures. Therefore, all of the studies were in English, which might lead to the language bias. Thirdly, all of the studies evaluated the efficacy and safety outcomes via comparing the combining use of the three anti-platelet agents (aspirin, thienopyridine and vorapaxar) with merely standard dual anti-platelet therapy (aspirin and thienopyridine). There was lack of trials on the effect about using vorapaxar alone, vorapaxar combined with aspirin, vorapaxar combined with thienopyridine. Lastly, the suitable therapeutic dose was still lack of evidence.
Conclusions
Vorapaxar is a novel anti-platelet drug which has a bright application prospect in patients with CAD. The results of this meta-analysis indicated that the combining use of vorapaxar, aspirin and thienopyridine may reduce the incidence of MI and ischemic stroke. However, it also increases the risk of bleeding events. Further studies are still needed before it is widely used in clinical practice.
Acknowledgements
Funding: This study was supported by grants from Science and Technology Planning Project of Guangdong Province, China (2014A020212088) and the Natural Science Foundation of Guangdong Province, China (No.S2013040014921).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Costopoulos C, Niespialowska-Steuden M, Kukreja N, et al. Novel oral anticoagulants in acute coronary syndrome. Int J Cardiol 2013;167:2449-55. [Crossref] [PubMed]
- Lee CW. Dual antiplatelet therapy for coronary artery disease. Circ J 2015;79:255-62. [Crossref] [PubMed]
- Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 2015;12:30-47. [Crossref] [PubMed]
- Oestreich J. SCH-530348, a thrombin receptor (PAR-1) antagonist for the prevention and treatment of atherothrombosis. Curr Opin Investig Drugs 2009;10:988-96. [Crossref] [PubMed]
- Magnani G, Bonaca MP, Braunwald E, et al. Efficacy and safety of vorapaxar as approved for clinical use in the United States. J Am Heart Assoc 2015;4:e001505. [Crossref] [PubMed]
- Becker RC, Moliterno DJ, Jennings LK, et al. Safety and tolerability of SCH 530348 in patients undergoing non-urgent percutaneous coronary intervention: a randomised, double-blind, placebo-controlled phase II study. Lancet 2009;373:919-28. [Crossref] [PubMed]
- Held C, Tricoci P, Huang Z, et al. Vorapaxar, a platelet thrombin-receptor antagonist, in medically managed patients with non-ST-segment elevation acute coronary syndrome: results from the TRACER trial. Eur Heart J Acute Cardiovasc Care 2014;3:246-56. [Crossref] [PubMed]
- Valgimigli M, Tricoci P, Huang Z, et al. Usefulness and safety of vorapaxar in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention (from the TRACER Trial). Am J Cardiol 2014;114:665-73. [Crossref] [PubMed]
- Tricoci P, Lokhnygina Y, Huang Z, et al. Vorapaxar with or without clopidogrel after non-ST-segment elevation acute coronary syndromes: results from the thrombin receptor antagonist for clinical event reduction in acute coronary syndrome trial. Am Heart J 2014;168:869-77.e1.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Goto S, Yamaguchi T, Ikeda Y, et al. Safety and exploratory efficacy of the novel thrombin receptor (PAR-1) antagonist SCH530348 for non-ST-segment elevation acute coronary syndrome. J Atheroscler Thromb 2010;17:156-64. [Crossref] [PubMed]
- Tricoci P, Huang Z, Held C, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med 2012;366:20-33. [Crossref] [PubMed]
- Scirica BM, Bonaca MP, Braunwald E, et al. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P-TIMI 50 trial. Lancet 2012;380:1317-24. [Crossref] [PubMed]
- Niazi AK, Dinicolantonio JJ, Lavie CJ, et al. Triple versus dual antiplatelet therapy in acute coronary syndromes: adding cilostazol to aspirin and clopidogrel? Cardiology 2013;126:233-43. [Crossref] [PubMed]
- Terpening C. An appraisal of dual antiplatelet therapy with clopidogrel and aspirin for prevention of cardiovascular events. J Am Board Fam Med 2009;22:51-6. [Crossref] [PubMed]
- Reaume KT, Regal RE, Dorsch MP. Indications for dual antiplatelet therapy with aspirin and clopidogrel: evidence-based recommendations for use. Ann Pharmacother 2008;42:550-7. [Crossref] [PubMed]
- Ben-Dor I, Kleiman NS, Lev E. Assessment, mechanisms, and clinical implication of variability in platelet response to aspirin and clopidogrel therapy. Am J Cardiol 2009;104:227-33. [Crossref] [PubMed]
- Moudgil R, Al-Turbak H, Osborne C, et al. Superiority of ticagrelor over clopidogrel in patients after cardiac arrest undergoing therapeutic hypothermia. Can J Cardiol 2014;30:1396-9. [Crossref] [PubMed]
- Goto S, Tomita A. New antithrombotics for secondary prevention of acute coronary syndrome. Clin Cardiol 2014;37:178-87. [Crossref] [PubMed]
- de Souza Brito F, Tricoci P. Novel anti-platelet agents: focus on thrombin receptor antagonists. J Cardiovasc Transl Res 2013;6:415-24. [Crossref] [PubMed]
- Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 2005;3:1800-14. [Crossref] [PubMed]
- Kato Y, Kita Y, Hirasawa-Taniyama Y, et al. Inhibition of arterial thrombosis by a protease-activated receptor 1 antagonist, FR171113, in the guinea pig. Eur J Pharmacol 2003;473:163-9. [Crossref] [PubMed]
- Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012;366:1404-13. [Crossref] [PubMed]
- TRA*CER Executive and Steering Committees. The Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRA*CER) trial: study design and rationale. Am Heart J 2009;158:327-334.e4.
- Morrow DA, Scirica BM, Fox KA, et al. Evaluation of a novel antiplatelet agent for secondary prevention in patients with a history of atherosclerotic disease: design and rationale for the Thrombin-Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2 degrees P)-TIMI 50 trial. Am Heart J 2009;158:335-341.e3.
- Capodanno D, Bhatt DL, Goto S, et al. Safety and efficacy of protease-activated receptor-1 antagonists in patients with coronary artery disease: a meta-analysis of randomized clinical trials. J Thromb Haemost 2012;10:2006-15. [Crossref] [PubMed]