Association between rs1761667 polymorphism of CD36 gene and risk of coronary atherosclerosis in Egyptian population
Introduction
CD36 is a B scavenger receptor that is present in various types of cells as macrophage and platelet. Recent studies have demonstrated that CD36 is involved in the progression of atherosclerosis. Macrophage and platelet CD36 participate in atherosclerotic arterial lesion formation through its interaction with oxidized low-density lipoprotein (oxLDL) and promotes atherosclerotic inflammatory processes (1) and is involved in thrombus formation after atherosclerotic plaque rupture. An association of CD36 gene variant rs1761667 with developing coronary artery disease (CAD) have been detected (2).
There have been suggestions that polymorphisms of the CD36 gene modulate lipid metabolism and cardiovascular risk (CVR) (3), essential hypertension (4), insulin resistance, familial type 2 DM (5) and an elevation of body mass index (BMI) (6). Other studies have been shown that SNPs, rs1761667 has direct association with type 2 diabetes mellitus (T2DM) or metabolic syndrome (MetS) but do not have a direct association with CAD (7,8). The CD36 rs1761667 has not been investigated in Arab CAD patients. The only single study of the Egyptian population showed that CD36 rs1761667 SNP is positively associated with increased risk of MetS and its components (9), but did not investigate the relationship between it and CAD.
Therefore, our study selected SNPs, rs1761667 as candidate SNPs to investigate whether there is an association between the genetic and functional effects of CD36 gene polymorphisms on CAD development in the Sohag population of Egypt. Which may lead to a new treatment strategy for atherosclerosis.
Methods
Study subjects
This case-control study was conducted on patients with coronary atherosclerosis attending Sohag University Hospital, Egypt from October 2014 to June 2015. We initially enrolled a total of 100 consecutive patients with coronary atherosclerosis and 100 healthy controls in the study. Clinical diagnosis of coronary atherosclerosis was evaluated by percutaneous coronary angiography, reviewed by two experienced cardiologists. The inclusion criteria for patients in the CAD group included: (I) older than 18 years of age; (II) a diagnosis of CAD according to the World Health Organization (WHO) CAD diagnostic criteria set in 1979; and (III) a stenosis degree greater than or equal to 50% in at least one artery determined by angiography. Healthy control subjects, without coronary atherosclerosis, were selected in the same period and from the same hospital, and were frequency matched to the cases by age and gender. All controls were individuals free of CAD, extreme care was taken to exclude CAD that determined by medical history, clinical examinations, electrocardiography (ECG), stress test ECG, echocardiography or dobutamine stress echocardiography if indicated. The case exclusion criteria included patients with: (I) systemic diseases such as inflammation, rheumatic autoimmune diseases, tumor, type 1 diabetes mellitus, thyroid dysfunction (current hypo- or hyperthyroidism), liver and kidney diseases and (II) those with coronary myocardial bridge were excluded from the study. At enrollment, demographic characteristics, anthropometric measures, medical histories were collected from each subject. Written informed consent was obtained from all enrolled participants and this study and the study protocol was reviewed and approved by Scientific and Ethical committees of the Sohag Faculty of Medicine.
Peripheral blood was collected using EDTA-anticoagulant tubes from 100 patients and 100 healthy controls for DNA extraction. We excluded 29 cases from case group (19-blood collection was small to complete investigation, 2-failure of DNA extraction, 5-hemolysis, 3-DNA degradation) and 24 from control group (13-blood collection was small to complete investigation, 4-failure of DNA extraction, 3-failure of enzyme digestion, 3-hemolysis, 1-DNA degradation) so, the CAD group included 71 patients, while the control group included 76 participants. The SNP rs1761667 of CD36 gene was genotyped using real-time polymerase chain reaction (PCR) and allele discrimination technique.
Laboratory tests
Laboratory investigations were carried out using fully automated spectrophotometer Beckman coulter AU480 (Beckman coulter Diagnostics, Washington, USA). Blood samples were drawn for measurement of serum levels of total cholesterol (TC) (mg/dL), Triglycerides (TG) (mg/dL), high-density lipoprotein (HDL-C) (mg/dL), and LDL-C (mg/dL) after a 12-hour overnight fast.
Flow cytometry
Flow cytometry was done on coulter Epics XL 4-color flow cytometer (coulter, electronics, Hialeah, FL, USA) to determine the expression of CD36 using monoclonal antibodies, cells were considered positive for CD36 when 20% or more of the gated cells were expressing it. Fluorescein isothiocyanate (FITC) -conjugated anti-human CD36, R-PE-conjugated antihuman CD14 and FITC-mouse anti-human IgG (isotype control) supplied by BD Pharmingen were used. Gating was done on the monocyte population based on forward and side scatter properties. Samples of whole blood (100 μL) were stained doubly with 10 μL FITC-anti-human CD36 and 10 μL PE-anti-human CD14 (a specific marker for monocytes) for 20 minutes at room temperature and protected from light. The cells were then washed three times with phosphate-buffered saline-EDTA solution. After washing, FACS lysing solution (Becton Dickinson) was added and mixed together. The mixture was left at room temperature for 30 minutes to lyse erythrocytes and then analyzed. The MFI of CD36 on CD14-positive monocytes was measured using CellQuest 1.1 software.
Genotyping
Genomic DNA was extracted from peripheral blood leucocytes using the commercially available Spin-column technique kit for DNA extraction from human whole blood (The PureLink® Genomic DNA Mini Kit, Invitrogen™, CA K1820-01, Lot 1607711, Carlsbad, CA 92008, USA). The extracted DNA samples were stored at −20 °C for future use. Determination of CD36 gene polymorphism was carried out by real-time PCR (gene discrimination) (Applied Biosystems, The StepOne™ Real-Time PCR System, Foster City, CA, USA), using primers (Life Technologies, 5791 Van Allen Way, Carlsbad, CA 92008, USA) with the following sequence: sense, 5'A-TCGTATCCTGTCACTCCTCCAA-3' and Anti-sense: 5'GCTATCATGGAAACCAAGAAAGAC-3'. The PCR-mixture is composed of distilled water (7.72 μL), VIC probe (0.44 μL), FAM probe (0.44 μL), sense and anti-sense probes (0.20 μL each), Universal Master Mix (AmpliTaq Gold® 360 PCR Master Mix, Foster City, California, CA 94404, USA) (11.0 μL), and template DNA (2.0 μL) to reach a total volume of 22 μL. After an initial step of 2 min at 50 °C and 10 min at 95 °C to activate the AmpliTaq Gold, the products were amplified using 40 cycles of 15 seconds at 95 °C and one minute at 62 °C. Then, allele detection and genotyping calling were performed using Rotor-GeneTM 6000 (Corbett Research, Mortlake, New South Wales, Australia) with the available installed software.
Statistical analysis
All continuous variables were statistically described in terms of mean ± standard deviation (±SD). Categorical variables were described with absolute and relative (percentage) frequencies. P values were used to describe significance. Statistical significance was set as a P value <0.05. Hardy-Weinberg equilibrium was tested using a χ2 test to judge the reliability of the gene frequency. The strength of association of SNP of rs1761667 polymorphism with CAD was determined by Odds ratios (ORs) with 95% confidence intervals (CIs) using Logistic Regression Analysis and to estimate the significance of differences in the risk. Multivariate Logistic regression analysis was used to examine the association of clinical and laboratory parameters with different genotype of the SNP of rs1761667 polymorphism with CAD. All statistical calculations were done using Statistical Package for Social Sciences (SPSS for Windows) software (version 22.0, SPSS Inc., Chicago, IL, USA).
Results
Our study population consisted of 71 cases and 76 healthy controls. Cases and controls were comparable with respect to age and gender. Cases were more probably to smoke cigarettes (65% vs. 45%), have diabetes (41% vs. 26%) and hypertension (48.7% vs. 38.7%). The percent of family history of CAD, dyslipidemia, BMI, waist circumference, MetS and its components in the patient group were significantly higher than that of the control group (P<0.001). Besides, cases have significant low ejection fraction (EF) and lower levels of serum HDL-C and higher levels of serum LDL-C than that in controls (P<0.001, P=0.006, P<0.001 respectively). Characteristics of the study subjects are shown in Table 1.
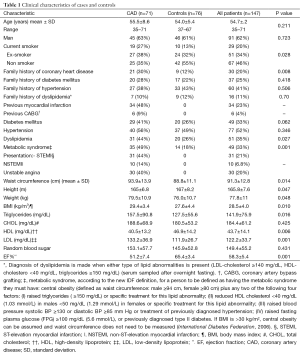
Full table
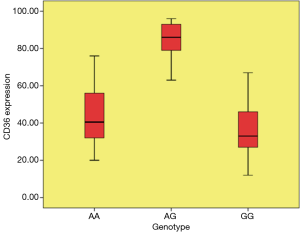
The distribution of rs1761667 genotype & CD36 expression frequencies in patient and control groups are presented in Table 2. RFLP analysis confirmed that there were three genotypes (GG, AG, and AA) for rs1761667 (Figure 1A) in the chipping fragments. The distribution of rs1761667 genotypes between the two groups was significantly different (P<0.001), with the AG genotype being significantly higher in the CAD group than in the control group (P<0.001, Figure 1A). There were no significant differences between the two groups in the allele frequencies of rs1761667. The results of direct sequencing were consistent with identifications made by agarose gel electrophoresis. CD36 expression in participants was detected by flowcytometry after we genotyped 147 participants for the 3 genotypes of rs1761667. The expression level of CD36 in the CAD group was significantly higher than that in the control group (P<0.001), with significant differences in the CAD patients with an AG genotype of rs1761667 compared with those with an AA and GG genotype (P<0.001, Figure 1B). The plasma levels (mg/dL) of LDL in the CAD group were much higher than that in the control group (P<0.001). Furthermore, the plasma LDL levels in CAD patients were significantly different in patients with the three different genotypes (P=0.046).
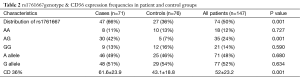
Full table
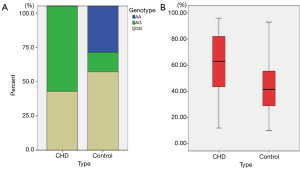
Distribution of CD36 genotypes in the patient group was AA (n=8), AG (n=30), and GG (n=9) (Figure 2), while in the control group it was AA (n=10), AG (n=5), and GG (n=12). AG genotype was significantly more prevalent among T2DM, STEMI, MetS and its components patients (P<0.05). Patients with genotypes AG had significantly higher BMI, wider waist circumstance, and higher degree of dyslipidemia (LDL-C) than patients with genotype AA and GG (P<0.05). The OR for the high risk AG genotype is 7.75 with 95% CI from 2.48–24.25 (P<0.001). Patients with genotypes AG had significantly higher CD36 expression (P<0.001) than patients with genotype AA and GG.
We investigated the associations of rs1761667 genotypes with clinical indexes in the CAD group and we observed significant differences in HDL, LDL and BMI in CAD patients with AG genotype of rs1761667 (all P<0.05, Table 3). And no differences in TG, TC, height, weight or EF in CAD patients with AG genotype.
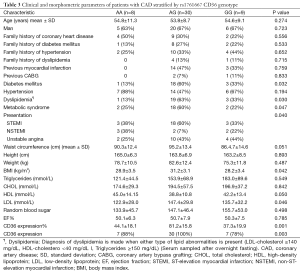
Full table
We screened the risk factors of CAD and predicted its occurrence with logistic regression analysis. Genotypes of rs1761667 were considered, as well as traditional risk factors of CAD such as age, sex, BMI, hypertension, dyslipidemia, T2DM, MetS, smoking history and family history of CAD, TG, TC, HDL, and LDL. The results showed that current smoking, family history of CAD, MetS, LDL and the AG genotype of rs1761667 factored into the final equation (P<0.05) (Table 4). The predictive equation was established according to the parameters in Table 4. The associations of the AG genotype of rs1761667genotype and CAD remained statistically significant after further adjustment for smoking, family history of CAD, MetS, LDL. The sensitivity, specificity, +ve Likelihood ratio and −ve Likelihood ratio of the AG genotype of rs1761667 were 42.3, 93.4, 6.4 (moderate/ often useful test), 0.61 (very small/ rarely useful test), respectively.
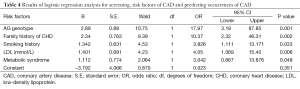
Full table
Effect of rs1761667 polymorphism on CAD is presented in Table 5 which shows that the AG genotype could increase the risk of CAD in both unadjusted and adjusted logistic regression models (unadjusted OR=7.75, 95% CI, 2.48–24.25, P<0.001; adjusted OR=17.97, 95% CI, 3.19–87.85, P=0.001).
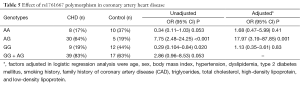
Full table
Discussion
There is no clear indication whether mutations of the CD36 receptor gene protect against or increase the risk of hypercholesterolemia, atherosclerosis, and its complications such as vascular dysfunction, progression of arterial hypertension, and ischemic heart disease. Considering the pivotal role of CD36 in T2DM, MetS, lipid metabolism (7,8,10) and the association of CD36 gene variants rs1761667 with developing CAD (2) prompted us to investigate its SNP association with CAD in our Sohag population. In the present study, we evaluated the associations between polymorphisms of the rs1761667 in the CD36 gene and the susceptibility to CAD in 71 CAD patients and 76 healthy controls in the Sohag population of Egypt. We found significant differences in the genotype distribution of rs1761667 between the CAD and control groups, with a significantly higher frequency of the AG genotype in the CAD group compared to the control group. Our results are similar to those reported in previous studies (2,6).
Another key finding of our study is that the plasma levels of LDL in patients with CAD were higher than those of controls and were related to the genotypes of rs1761667. Evidence from the literature has shown that foam cell formation is the initial critical step of atherosclerosis. The atherogenic process starts after the transmigration of blood-circulating monocytes into the arterial intima, where they differentiate into macrophages, which bind and internalize oxLDL through CD36. The internalized oxLDL provides its specific oxidized lipids as ligands for the nuclear hormone receptor peroxisome proliferator–activated receptor (PPAR-γ) and upregulates the expression of CD36 and facilitates further uptake of oxLDL (11). The interaction between CD36 and oxLDL also induces the secretion of cytokines that recruit immune cell infiltrates in the arterial intima (12) and the arterial inflammation provoked by foam cells induces arterial narrowing, establishing atherosclerotic vascular diseases, so we evaluated the relationship of rs1761667 polymorphism in the CD36 gene with LDL. The results of some studies showed that LDL concentrations were higher in CAD individuals than in non-CAD individuals (2,12), and our findings were similar. However, there have been few reports on the association of plasma LDL levels with CD36 polymorphisms in CAD patients. We found that CAD patients with an AG genotype had higher plasma LDL levels than those with GG or AA genotypes (P=0.046). This demonstrates that plasma LDL level and rs1761667 polymorphism have a close relationship. So, we can conclude that the AG genotype of the rs1761667 polymorphism in the CD36 gene may be involved in increasing the risk of cardiovascular disease and CAD pathogenesis.
These findings indicated that rs1761667 polymorphism may be closely associated with the risk of developing CAD in the Sohag population of Egypt, and that the AG genotype may be a genetic susceptibility factor for CAD. To our knowledge, this is the first time that an association has been reported between rs1761667 polymorphism in the CD36 gene and CAD in Egyptian patients, particularly in relation to the AG genotype in rs1761667.
Associations between rs1761667 polymorphism in the CD36 gene and diseases have been addressed previously. A recent study on the variants of the CD36 gene showed that SNP, rs1761667-A allele, associates with high oral fat detection thresholds in some obese subjects (13) and results suggested that individuals having a GATTC1 haplotype might be at risk of developing T2DM (14), therefore, might be susceptible to related complications. Moreover, the presence of A, G, and G alleles of SNPs rs1761667 (G>A), rs3211938 (T>G), and rs1984112 (T>G) tends to have increased BMI (15) and our findings were similar. The authors investigated patients with T2DM stratified into CAD-positive cases and CAD-negative controls and the results suggest that all of those T2DM in patients with CAD were higher than those of controls and were related to the genotypes of rs1761667 (P<0.001). We found that the CD36 SNP rs1761667 variant AG was significantly associated with higher BMI, as compared to variants AA and GG (P<0.015, P=0.54 and P=0.95, respectively). In harmony with the data were collected from the Tampere adult population CVR study (TAMRISK) ((15. Another study on Egyptian population showed that CD36 rs1761667 SNP is positively associated with increased risk of MetS and its components with genotype AG heterozygotes showing highest frequency among MetS patients (9). In accordance with the previously mentioned studies, we found that patients with genotypes AG had significantly higher, wider waist circumstance, and lower level of HDL (P<0.05) than patients with genotype AA and GG. Contrary to this study, no association of this CD36 variation with hypertension was found.
It has been demonstrated that the CD36 is a protein associated with uptake of oxidized forms of LDL and the SNP, rs1761667 A/G in the CD36 gene is correlated with increased consumption of total fat. A recent study has suggested that the A allele of cluster of differentiation CD36 SNP 1761667 associates with decreased lipid taste perception in obese Tunisian women (16). Another study on adherence to the Mediterranean diet (MD) in a high CVR population showed that adherence to MD modulates the effect of the CD36-rs1761667 polymorphism on plasma free fatty acids (FFA) concentration and BMI in a high CVR population (17). In accordance with the previously mentioned study that has been shown the benefits of the Mediterranean diet in secondary prevention of cardiovascular disease and these dietary patterns have been often studied with nutrigenetic approach, however, are often limited to European populations, making it difficult to generalize to different populations, so many randomized clinical trial with a nutrigenetic approach take place now to study the effect of polymorphisms in CD36 and STAT3 genes and different dietary interventions among patients with CAD (GENUTRI).
In spite of rs1761667 polymorphism affects lipid metabolism and oral fat perception and the genotype AG was found to be more prevalent among T2DM and MetS patients (7,8), the association of rs1761667 polymorphism with CAD remains to be illustrated. In agreement with the previously mentioned study (2), we found that genotype distributions of rs1761667 polymorphism in the CAD group significantly higher than controlled group. Antithesis to those studies (7,8). Our clinical results are consistent with strict inclusion criteria, our subjects were actual patients with CAD. We found that the genotype distributions of rs1761667 in the CAD and control groups were significantly different (P<0.001), with the frequency of the AG genotype being significantly higher in the CAD group (P<0.001). No significant difference was observed in the allele frequencies of G/A between the two groups (P=0.68). After adjustments for age, sex, BMI, hypertension, dyslipidemia, T2DM, smoking history, family history of CAD, TG, TC, HDL, and LDL in logistic regression, the results still indicated that the AG genotype of rs1761667 correlated with an increased risk of CAD (OR=17.97, 95% CI, 3.19–87.85, P=0.001). Logistic regression analysis showed that the AG genotype of rs1761667 was an independent risk factor for CAD. Furthermore, we found that rs1761667 polymorphism was closely related to CD36 expression and LDL plasma levels. These results indicated that polymorphism of rs1761667 may be associated with the risk of CAD in Sohag population of Egypt and that the AG genotype may be a genetic susceptibility factor for patients with CAD. This finding is useful for understanding the mechanistic basis of this genetic factor. To the best of our knowledge, this is the third study to report on an association between rs1761667 polymorphism and CAD.
Researchers have found that CD36 expression in monocytes is increased in patients with CAD and that this could reflect the severity of coronary artery atherosclerosis to a certain extent (18). The exact mechanisms which may link between CD36 genetic variability and atherosclerosis can conclude in several. First, CD36 is a cell membrane long-chain polyunsaturated fatty acid transporter in a wide variety of metabolically active tissues, including muscle, heart, and adipocytes (19). Impaired function of CD36 could decrease the intramuscular fatty acid oxidation rate and thus increase the availability of fatty acids for storage in adipocytes (20). Second, experimental studies have shown that activated macrophages secrete various factors that inhibit the formation of mature adipocytes. A CD36 dysregulation in infiltrated macrophages within adipose tissue could alter the signaling pathway that feeds back to control adipocyte expansion (21). Third, CD36 plays a key role in the activation PPAR-γ, a nuclear receptor responsible for adipocyte differentiation and adipogenesis (22). Thus, an alteration in CD36 may impact on PPAR-у mediated adipocyte differentiation.
So, many researchers suggested that CD36 expression in circulating monocytes may be a marker for CAD (23). Therefore, we evaluated the association between CD36 expression and three genotypes of the rs1761667 allele in CAD patients and the control group. The expression level of CD36 in the CAD group was significantly higher than that in the control group (P<0.001). Furthermore, we compared CD36 expression among CAD patients carrying different genotypes of rs1761667 and found that the CD36 expression in the CAD patients with an AG genotype was remarkably higher than in those with the AA and GG genotypes (P<0.001). This result indicated that rs1761667 polymorphism seems to be involved in CAD pathogenesis. Our results are similar to those previous publications (2.23).
We investigated about the relationship between rs1761667 polymorphism in the CD36 gene with CD36 expression and CAD and we found many studies which may provide a possible mechanism. Researchers suggested that the recently identified soluble CD36 (sCD36) was proposed to reflect tissue CD36 expression and may thus be a potential marker integrating hyperglycemia and atherosclerosis (24). The researchers also found that sCD36 was increased in subjects with impaired glucose regulation, MetS or increased likelihood of fatty liver and intima-media thickness. So, they suggested that plasma sCD36 could be used as markers of atherosclerosis, insulin resistance and fatty liver in a nondiabetic healthy population (25). The exact mechanisms which may link rs1761667 polymorphism in the CD36 gene with CD36 expression to coronary atherosclerosis need further investigation. But the linkage between rs1761667 polymorphisms, CD36 expression and plasma LDL and HDL-C level may provide a possible mechanism that merits further investigation.
In our study, CAD group carrying the AG genotype had significant lower level of HDL-C (38.8±10.8 mg/dL) compared with subjects with AA or GG genotype (45.0±14.15, 42.2±13.4 mg/dL) and had a significant higher level of LDL-C (147.4±29.8 mg/dL) compared with subjects with AA or GG genotype (122.9±28.0, 135.7±32.2 mg/DL) which was consistent with previous reports. Besides, we found AG genotype conferred a significant elevated risk of coronary atherosclerosis (OR=7.752, 95% CI, 0.48–24.25, P<0.001) and this association was also suggested by another study (2). In contrast Ramos-Lopez et al reported that the A allele of CD36 was predominant in subjects from West Mexico. In addition, a high-fat diet and high serum cholesterol levels were associated with the A/A genotype (26).
We conclude that the AG genotype of the rs1761667 polymorphism in the CD36 gene may be involved in CAD pathogenesis and the association of this genetic factor is not restricted to CAD, but is also related to increase BMI, T2DM, MetS and its components so, the genetic risk factors should not be neglected. In addition, the limitation of this study was the relatively small sample size, which hampered our ability to detect more significant associations.
Acknowledgements
The authors would like to thank all members of the Internal Medicine and Clinical Pathology Department, Sohag faculty of Medicine for their help and support throughout this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nicholson AC, Han J, Febbraio M, et al. Role of CD36, the macrophage class B scavenger receptor, in atherosclerosis. Ann N Y Acad Sci 2001;947:224-8. [Crossref] [PubMed]
- Zhang Y, Ling ZY, Deng SB, et al. Associations between CD36 gene polymorphisms and susceptibility to coronary artery heart disease. Braz J Med Biol Res 2014;47:895-903. [Crossref] [PubMed]
- Ma X, Bacci S, Mlynarski W, et al. A common haplotype at the CD36 locus is associated with high free fatty acid levels and increased cardiovascular risk in Caucasians. Hum Mol Genet 2004;13:2197-205. [Crossref] [PubMed]
- Liu X, Meng F, Yang P. Association study of CD36 single nucleotide polymorphisms with essential hypertension in the Northeastern Han Chinese. Gene 2013;527:410-5. [Crossref] [PubMed]
- Leprêtre F, Vasseur F, Vaxillaire M, et al. A CD36 nonsense mutation associated with insulin resistance and familial type 2 diabetes. Hum Mutat 2004;24:104. [Crossref] [PubMed]
- Yun YM, Song EY, Song SH, et al. CD36 polymorphism and its relationship with body mass index and coronary artery disease in a Korean population. Clin Chem Lab Med 2007;45:1277-82. [Crossref] [PubMed]
- Banerjee M, Gautam S, Saxena M, et al. Association of CD36 gene variants rs1761667(G.A) and rs1527483(C.T) with Type 2 diabetes in North Indian population. Int J Diabetes Mellit 2010;2:179-83. [Crossref] [PubMed]
- Noel SE, Lai CQ, Mattei J, et al. Variants of the CD36 gene and metabolic syndrome in Boston Puerto Rican adults. Atherosclerosis 2010;211:210-5. [Crossref] [PubMed]
- Bayoumy NM, El-Shabrawi MM, Hassan HH. Association of cluster of differentiation 36 gene variant rs1761667 (G>A) with metabolic syndrome in Egyptian adults. Saudi Med J 2012;33:489-94. [Crossref] [PubMed]
- Keller KL, Liang LC, Sakimura J, et al. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity (Silver Spring) 2012;20:1066-73. [Crossref] [PubMed]
- Endemann G, Stanton LW, Madden KS, et al. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem 1993;268:11811-6. [Crossref] [PubMed]
- Koenig W, Karakas M, Zierer A, et al. Oxidized LDL and the risk of coronary heart disease: results from the MONICA/KORA Augsburg Study. Clin Chem 2011;57:1196-200. [Crossref] [PubMed]
- Sayed A, Šerý O, Plesnik J, et al. CD36 AA genotype is associated with decreased lipid taste perception in young obese, but not lean, children. Int J Obes (Lond) 2015;39:920-4. [Crossref] [PubMed]
- Gautam S, Agrawal CG, Banerjee M. CD36 gene variants in early prediction of type 2 diabetes mellitus. Genet Test Mol Biomarkers 2015;19:144-9. [Crossref] [PubMed]
- Solakivi T, Kunnas T, Nikkari ST. Contribution of fatty acid transporter (CD36) genetic variant rs1761667 to body mass index, the TAMRISK study. Scand J Clin Lab Invest 2015;75:254-8. [Crossref] [PubMed]
- Mrizak I, Šerý O, Plesnik J, et al. The A allele of cluster of differentiation 36 (CD36) SNP 1761667 associates with decreased lipid taste perception in obese Tunisian women. Br J Nutr 2015;113:1330-7. [Crossref] [PubMed]
- Ortega-Azorín C, Godoy D, Carrasco P, et al. Mediterranean diet modulates the effect of rs1761667 in the CD36 gene on FFA concentration and BMI in a high cardiovascular risk population. Endocrine Abstracts 2013;32:179.
- Piechota M, Banaszewska A, Dudziak J, et al. Highly upregulated expression of CD36 and MSR1 in circulating monocytes of patients with acute coronary syndromes. Protein J 2012;31:511-8. [Crossref] [PubMed]
- Ibrahimi A, Abumrad NA. Role of CD36 in membrane transport of long-chain fatty acids. Curr Opin Clin Nutr Metab Care 2002;5:139-45. [Crossref] [PubMed]
- Bonen A, Parolin ML, Steinberg GR, et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 2004;18:1144-6. [Crossref] [PubMed]
- Lacasa D, Taleb S, Keophiphath M, et al. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology 2007;148:868-77. [Crossref] [PubMed]
- Tontonoz P, Nagy L, Alvarez JG, et al. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998;93:241-52. [Crossref] [PubMed]
- Maiwald S, Zwetsloot PP, Sivapalaratnam S, et al. Monocyte gene expression and coronary artery disease. Curr Opin Clin Nutr Metab Care 2013;16:411-7. [Crossref] [PubMed]
- Handberg A, Levin K, Højlund K, et al. Identification of the oxidized low-density lipoprotein scavenger receptor CD36 in plasma: a novel marker of insulin resistance. Circulation 2006;114:1169-76. [Crossref] [PubMed]
- Handberg A, Højlund K, Gastaldelli A, et al. Plasma sCD36 is associated with markers of atherosclerosis, insulin resistance and fatty liver in a nondiabetic healthy population. J Intern Med 2012;271:294-304. [Crossref] [PubMed]
- Lopez-Ramos O, Panduro A, Martinez-Lopez E, et al. Genetic Variant in the CD36 Gene (rs1761667) is Associated with Higher Fat Intake and High Serum Cholesterol among the Population of West Mexico. J Nutr Food Sci 2005;5:1-5.


