
Diagnostic performance of quantitative flow ratio, non-hyperaemic pressure indices and fractional flow reserve for the assessment of coronary lesions in severe aortic stenosis
Introduction
Evaluation of coronary artery disease is an important consideration when assessing patients for transcatheter aortic valve replacement (TAVR). Fractional flow reserve (FFR) may be used to assess the physiological significance of coronary stenoses in the setting of severe aortic stenosis, and its usage to guide myocardial revascularisation has been associated with improved clinical outcomes when compared to angiographic guidance (1).
However, it would be desirable to avoid the administration of vasoactive medications in this vulnerable patient cohort (2). Furthermore, while non-hyperaemic indices are an alternative tool for physiological assessment in the setting of aortic stenosis (3,4), it would be advantageous to assess coronary lesions without the usage of wire-based tools.
One emerging technology for the physiological assessment of coronary stenoses is quantitative flow ratio (QFR), which is derived using complex mathematical methods built upon the principles of computational fluid dynamics (CFD) (5). QFR is computed using a modelled hyperaemic flow velocity, derived from thrombolysis in myocardial infarction frame count analysis, without pharmacologically-induced hyperaemia.
In this study we wished to compare the diagnostic performance of QFR against FFR and non-hyperaemic indices in the setting of severe aortic stenosis. We present the following article in accordance with the STARD reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-574/rc).
Methods
A single centre prospective study was performed at Monash Medical Centre, Melbourne between November 2018 and November 2019 on consecutive patients with symptomatic severe aortic stenosis who were being considered for TAVR. Full inclusion and exclusion criteria and flow of participants have been reported in the CAST-FFR study (6). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was reviewed by Monash Health Human Research Ethics Committee (HREC/43524/MonH-2018-67705v1) and all participants provided informed consent for inclusion in the study.
Pressure wire assessment
Coronary angiography was performed using standard protocols. Angiography was acquired at 15 frames per second. Pressure wire assessment (PressureWire X, Abbott Laboratories, Abbott Park, IL) was performed on coronary lesions of 30–90% severity in vessels with ≥2 mm diameter. Intracoronary glyceryl trinitrate (100 µg) was administered. The pressure wire was equalised with aortic pressure and then positioned in the distal third of the artery, at least 20 mm beyond the coronary lesion. Measurements was recorded (QUANTIEN, Abbott Laboratories) at rest and then hyperaemia was induced with intravenous adenosine (140 µg/kg/min). Measurements were repeated if there was >0.02 drift in the distal arterial pressure/arterial pressure (Pd/Pa) at the guiding catheter tip. Haemodynamic recordings were exported to Python v3.8 (Python Software Foundation, Wilmington, DE) to calculate FFR, instantaneous wave-free ratio (iFR), diastolic pressure ratio during the wave-free period (dPR) and Pd/Pa, using previously described methods (7).
Quantitate flow ratio analysis
QFR analysis was performed using QAngio XA3D v3.1.1 (Medis Medical Imaging System, Leiden, The Netherlands) by an independent core laboratory, using previously described methods (8). Analysis was performed on two angiographic acquisitions that were separated by ≥25°, ensuring that the angiographic projections had minimal foreshortening of the stenosis, and only minimal overlap of the main vessel and the side branches. Two-dimensional quantitative coronary angiography (QCA) was performed, and percentage diameter stenosis and lesion length recorded. The pressure wire recordings and angiographic information which was used to perform the QFR modelling were undertaken contemporaneously.
Statistical analysis
Statistical analysis was performed using SPSS v26.0 (IBM Corporation, Armonk, New York, USA). Continuous variables are presented as mean ± standard deviation and categorical variables as frequencies (percentage). Correlation was assessed using a Pearson correlation coefficient (r). Agreement was assessed using a Bland-Altman technique. Discriminatory power was tested using the area under the receiver operating characteristic curve (AUC). Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were calculated. We calculated that with a functionally significant FFR prevalence of 33%, 68 vessels would be required to provide an 80% power to demonstrate an AUC of 0.70, with a type I error rate of 5%. Assuming that most patients would have two evaluable vessels, we calculated that 34 patients would be required for study inclusion. A two-sided P value of <0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 35 patients were included in the study. Baseline characteristics demonstrated an elderly population with a high prevalence of traditional cardiovascular risk factors (Table 1). Echocardiographic parameters demonstrated a mean aortic valve gradient of 44.3±11.8 mmHg and a mean aortic valve area of 0.91±0.22 cm2.
Table 1
| Characteristic | Values (total n=35) |
|---|---|
| Age, years | 75.5±6.5 |
| Male, n (%) | 25 [71] |
| Body mass index (kg/m2) | 28.7±7.1 |
| STS score (%) | 2.8±2.0 |
| Cardiovascular risk factors, n (%) | |
| Diabetes mellitus | 20 [57] |
| Hypertension | 24 [69] |
| Hyperlipidaemia | 23 [66] |
| Smoking history | 16 [46] |
| Family history of IHD | 9 [26] |
| Previous MI, n (%) | 4 [11] |
| Previous CVA or TIA, n (%) | 4 [11] |
| Peripheral vascular disease, n (%) | 1 [3] |
| Atrial fibrillation, n (%) | 4 [11] |
| Chronic kidney disease, n (%) | 3 [9] |
| Echocardiographic parameters | |
| Left ventricular ejection fraction (%) | 64.1±8.7 |
| Peak aortic valve velocity (m/s) | 4.3±0.5 |
| Mean aortic valve gradient (mmHg) | 44.3±11.8 |
| Aortic valve area (cm2) | 0.91±0.22 |
CVA, denotes cerebrovascular accident; IHD, ischaemic heart disease; MI, myocardial infarction; STS, Society of Thoracic Surgeons; TIA, transient ischaemic attack.
Pressure wire assessment
The mean FFR was 0.83±0.10 and 22 vessels (39%) had a functionally significant FFR ≤0.80. The mean iFR and dPR were 0.83±0.12 and 0.86±0.11, respectively and 51% and 49% had functionally significant values ≤0.89. The mean Pd/PA was 0.91±0.06 and 54% had significant lesions ≤0.92. No adverse events were recorded during pressure wire assessment.
Quantitate flow ratio analysis
A total of 68 vessels were considered for QFR analysis, but 11 vessels were excluded as there were inadequate orthogonal views, leaving a total of 57 vessels for inclusion. The most commonly assessed vessel was the left anterior descending artery (54%) (Table 2). The mean QFR was 0.83±0.12 and 33% of lesions had a functionally significant QFR ≤0.80. The mean QCA diameter stenosis was 33.6%±11.8% and QCA lesion length 9.5±6.6 mm.
Table 2
| Coronary artery | N (%) (N=57) |
|---|---|
| Left anterior descending artery | 31 [54] |
| Diagonal artery | 5 [9] |
| Ramus intermedius artery | 1 [2] |
| Left circumflex artery | 5 [9] |
| Obtuse marginal artery | 9 [16] |
| Right coronary artery | 3 [5] |
| Posterior descending artery | 3 [5] |
Diagnostic performance of QFR
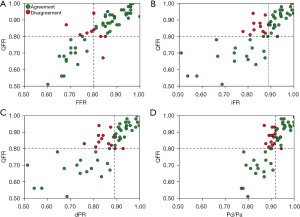
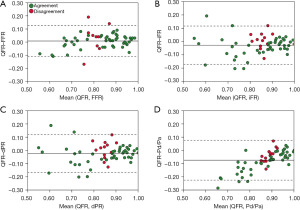
There was strong correlation between QFR and FFR [r=0.86; 95% confidence interval (CI): 0.78 to 0.92; P<0.001], iFR (r=0.80; 95% CI: 0.69 to 0.88; P<0.001), dPR (r=0.81; 95% CI: 0.69 to 0.88; P<0.001) and Pd/Pa (r=0.83; 95% CI: 0.72 to 0.89; P<0.001) (Figure 1). Bland-Altman analysis demonstrated agreement amongst QFR and FFR and non-hyperaemic indices (Figure 2).
QFR demonstrated an excellent discriminatory power to predict functionally significant FFR (AUC =0.92; 95% CI: 0.84 to 1.00; P<0.001) (Figure 3A) with good diagnostic performance [sensitivity, 73%, specificity 91%, positive predictive value (PPV) 84%, negative predictive value (NPV) 84%, accuracy 84%] (Tables 3,4). QFR demonstrated similar diagnostic performance to iFR (difference in AUC =0.04; 95% CI: −0.04 to 0.12; P=0.31), dPR (difference in AUC =0.04; 95% CI: −0.04 to 0.12; P=0.35) and Pd/Pa (difference in AUC =0.06; 95% CI: −0.01 to 0.14; P=0.11). QFR demonstrated superior diagnostic performance to QCA diameter stenosis (difference in AUC =0.24; 95% CI: 0.08 to 0.39; P=0.003) and lesion length (difference in AUC =0.37; 95% CI: 0.20 to 0.54; P<0.001).
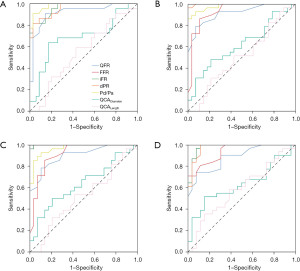
Table 3
| References | AUC (95% CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|
| FFR | ||||||
| QFR | 0.92 (0.84 to 1.00) | 73 | 91 | 84 | 84 | 84 |
| iFR | 0.96 (0.92 to 1.00) | 95 | 77 | 72 | 96 | 84 |
| dPR | 0.96 (0.91 to 1.00) | 91 | 77 | 71 | 93 | 82 |
| Pd/Pa | 0.98 (0.96 to 1.00) | 100 | 74 | 71 | 100 | 84 |
| QCAdiameter | 0.68 (0.53 to 0.83) | 14 | 94 | 60 | 63 | 63 |
| QCAlength | 0.55 (0.40 to 0.70) | 36 | 69 | 42 | 63 | 56 |
| iFR | ||||||
| QFR | 0.92 (0.85 to 0.99) | 62 | 96 | 95 | 71 | 79 |
| FFR | 0.93 (0.86 to 1.00) | 72 | 96 | 95 | 77 | 84 |
| dPR | 1.00 (0.99 to 1.00) | 97 | 100 | 100 | 97 | 98 |
| Pd/Pa | 0.98 (0.96 to 1.00) | 93 | 86 | 87 | 92 | 89 |
| QCAdiameter | 0.62 (0.47 to 0.77) | 86 | 4 | 48 | 20 | 46 |
| QCAlength | 0.51 (o.35 to 0.66) | 66 | 32 | 50 | 47 | 49 |
| dPR | ||||||
| QFR | 0.90 (0.83 to 0.98) | 61 | 93 | 89 | 71 | 77 |
| FFR | 0.92 (0.84 to 0.99) | 71 | 93 | 91 | 77 | 82 |
| iFR | 1.00 (0.99 to 1.00) | 100 | 97 | 97 | 100 | 98 |
| Pd/Pa | 0.97 (0.93 to 1.00) | 93 | 83 | 84 | 92 | 88 |
| QCAdiameter | 0.64 (0.49 to 0.78) | 14 | 97 | 80 | 54 | 56 |
| QCAlength | 0.52 (0.36 to 0.67) | 36 | 69 | 53 | 53 | 53 |
| Pd/Pa | ||||||
| QFR | 0.89 (0.80 to 0.97) | 58 | 96 | 95 | 66 | 75 |
| FFR | 0.94 (0.88 to 0.99) | 71 | 100 | 100 | 74 | 84 |
| iFR | 0.98 (0.95 to 1.00) | 87 | 92 | 93 | 86 | 89 |
| dPR | 0.98 (0.94 to 1.00) | 84 | 92 | 93 | 83 | 88 |
| QCAdiameter | 0.63 (0.48 to 0.77) | 13 | 96 | 80 | 48 | 51 |
| QCAlength | 0.58 (0.43 to 0.73) | 39 | 73 | 63 | 50 | 54 |
AUC, area under the receiver operating characteristic curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; FFR, fractional flow reserve; QCA, quantitative coronary angiography; QFR, quantitative flow ratio; iFR, instantaneous wave-free ratio; dPR, diastolic pressure ratio during the wave-free period; Pd/Pa, distal arterial pressure/arterial pressure.
Table 4
| Reference standard | QFR | Total | |
|---|---|---|---|
| ≤0.80 | >0.80 | ||
| FFR | |||
| ≤0.80 | 16 | 6 | 22 |
| >0.80 | 3 | 32 | 35 |
| Total | 19 | 38 | 57 |
| iFR | |||
| ≤0.89 | 18 | 11 | 29 |
| >0.89 | 1 | 27 | 28 |
| Total | 19 | 38 | 57 |
| dPR | |||
| ≤0.89 | 17 | 11 | 28 |
| >0.89 | 2 | 27 | 29 |
| Total | 19 | 38 | 57 |
| Pd/Pa | |||
| ≤0.92 | 18 | 13 | 31 |
| >0.92 | 1 | 25 | 26 |
| Total | 19 | 38 | 57 |
QFR, quantitative flow ratio; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; dPR, diastolic pressure ratio during the wave-free period; Pd/Pa, distal arterial pressure/arterial pressure.
QFR demonstrated an excellent discriminatory power to predict functionally significant iFR (AUC =0.92; 95% CI: 0.85 to 0.99; P<0.001) (Figure 3B) with acceptable diagnostic performance (sensitivity 62%, specificity 96%, PPV 95%, NPV 71%, accuracy 79%). QFR demonstrated an excellent discriminatory power to predict functionally significant dPR (AUC =0.90; 95% CI: 0.83 to 0.98; P<0.001) (Figure 3C) with acceptable diagnostic performance (sensitivity 61%, specificity 93%, PPV 89%, NPV 71%, accuracy 77%). QFR demonstrated a good discriminatory power to predict functionally significant Pd/Pa (AUC =0.89; 95% CI: 0.80 to 0.97; P<0.001) (Figure 3D) with acceptable diagnostic performance (sensitivity 58%, specificity 96%, PPV 95%, NPV 66%, accuracy 75%).
A total of 45 lesions (79%) had a QFR outside the borderline zone of 0.75 to 0.85 and for these lesions, QFR demonstrated an excellent discriminatory power to predict functionally significant FFR (AUC =0.93, 97% CI, 0.94 to 1.00; P<0.001), with excellent diagnostic performance (sensitivity 87%, specificity 97%, PPV 93%, NPV 94%, accuracy 93%). For lesion within the borderline zone, QFR did not demonstrate discriminatory power to predict functionally significant FFR (AUC =0.77; 95% CI: 0.53 to 1.00; P=0.08) and there was poor diagnostic performance (sensitivity 43%, specificity 60%, PPV 60%, NPV 43%, accuracy 50%).
Discussion
The key findings of this study are (I) QFR demonstrates excellent discriminatory power and good diagnostic performance for predicting functionally significant FFR in the setting of severe aortic stenosis; (II) QFR demonstrates an acceptable diagnostic performance for predicting functionally significant non-hyperaemic indices (iFR, dPR and Pd/PA); and (III) QFR demonstrates excellent diagnostic performance for predicting functionally significant FFR when QFR values are outside the borderline zone of 0.75 to 0.85.
Coronary artery disease is common in patients undergoing TAVR (9), but whether to revascularize these patients remains controversial and at present major society guidelines only recommend revascularisation for patients with significant (≥70%) proximal coronary artery disease or significant (≥50%) left main coronary artery disease (10).
Whilst there is a clear relationship between the severity of coronary artery disease and long-term clinical outcomes amongst TAVI patients (11), randomised trials have failed to demonstrate any improvement in clinical outcomes with angiographically-guided percutaneous coronary intervention (12). These findings are not surprising, however, as angiographically-guided PCI does not improve clinical outcomes for patients with stable coronary artery disease without aortic stenosis (13), and only FFR-guided PCI has been demonstrated to improve clinical outcomes (14,15).
FFR-guided PCI has been demonstrated in observational studies to be associated with improved clinical outcomes amongst TAVI patients (1) and a number of randomised clinical studies are currently evaluating the role of FFR-guided revascularisation, including the Revascularization in Patients Undergoing Transcatheter Aortic Valve Implantation (NOTION-3; NCT03058627) and Functional Assessment in TAVI (FAITAVI; NCT03360591) clinical studies.
Whilst FFR has for many years been considered the gold-standard for physiological assessment of coronary stenoses, equivalent clinical outcomes may also be achieved when PCI is guided by non-hyperaemic indices (16,17). This is a particularly attractive options for patients with severe aortic stenosis, in whom hyperaemia may be associated with significant hypotension. A number of studies have addressed the validity of non-hyperaemic indices in the setting of severe aortic stenosis (3,4,7,18), however their validity at long-term follow-up remains to be established (19).
Though non-hyperaemic indices are an attractive solution for assessing physiological significance, a less invasive option would be the avoidance of wire-based techniques and QFR has emerged as one potential technology to address this problem. The technology is well-validated in patients without aortic stenosis (20-24), including in patients with myocardial infarction (24-26), and growing evidence is building for its validation in aortic stenosis (27,28).
With this as a background, the present compared the diagnostic performance of QFR against not only FFR, but also multiple non-hyperaemic indices (iFR, dPR and Pd/Pa) in the setting of severe aortic stenosis. We confirmed that QFR demonstrated an excellent diagnostic performance against an FFR reference standard, with a diagnostic accuracy of 84%. Furthermore, QFR demonstrated an acceptable diagnostic performance against iFR, dPR and Pd/Pa reference standards, with a diagnostic accuracy of 79%, 77% and 75%, respectively.
Physiological assessment of coronary stenoses in the setting of severe aortic stenosis is challenging (29). Aortic stenosis is associated with blunting of systolic flow because of obstruction of ventricular emptying by the stenosed aortic valve and compression of the microcirculation by the contracting myocardium, elevating intraventricular pressure (3). Furthermore, the presence of left ventricular hypertrophy, elevated intraventricular pressure and microvascular dysfunction may attenuate the response of the microcirculation to hyperaemic agents. For these reasons, non-hyperaemic indices, in particular those that do not include the systolic phase of the cardiac cycle, may be more reliable in the setting of severe aortic stenosis. Adding to this complexity, FFR was originally validated against positron emission tomography in patients without severe aortic stenosis (30), and this work has not been replicated in patients with severe aortic stenosis, although validation of FFR and non-hyperaemic indices has been undertaken against single positron emission computed tomography (31).
In this study, we demonstrated that whilst QFR technology has been developed to predict FFR, QFR nonetheless demonstrated a discriminatory power to predict functionally significant lesions and an acceptable diagnostic accuracy when using a variety of non-hyperaemic indices (iFR, dPR and Pd/Pa) as reference standards. These results differ from a previous study which had demonstrated poor diagnostic accuracy (61%) when using pre-TAVI iFR as a reference standard (28). Lesion severity in our study (34%±12%) was less than what was reported in the prior work (52%±12%) and aortic valve area was greater (0.91±0.22 cm2) than what was previously reported (0.54±0.20 cm2) and this could potentially explain the discrepancy in diagnostic accuracy between these two studies. However, other groups have demonstrated that QFR has good agreement with iFR (32), consistent with our study findings.
In this study we demonstrated that QFR had excellent diagnostic performance (accuracy 93%) when QFR values were outside the borderline zone (0.75 to 0.85) but poor diagnostic performance (accuracy 50%) when QFR values were within the borderline zone. These results are consistent with recently published findings in patients without aortic stenosis (24) and highlight how a tool such as QFR could be incorporated into routine TAVI assessment. We would suggest that for patients with coronary stenoses of 30 90% that real-time QFR could be calculated at the time of diagnostic angiography. For patients whose QFR values lie within the borderline zone of 0.75 to 0.85, further invasive assessment of coronary stenoses could be undertaken, potentially using a hybrid iFR/FFR strategy as has previously been proposed (33), noting that optimal iFR/FFR thresholds for identifying functional significance may be different in the setting of severe aortic stenosis (18). This information could then be used by the Heart Team to help guide patient treatment decisions.
In this study, QCA diameter stenosis demonstrated poor discriminatory power to predict functionally significant FFR and furthermore, QCA lesion length demonstrated no discriminatory power to predict functionally significant FFR. These findings are consistent with studies performed in patients without severe aortic stenosis and stresses the importance of physiological assessment of coronary artery disease (34).
Moving forward, the role of QFR-guided revascularisation of patients will need to be assessed prospectively and the FAVOR4-QVAS (NCT03977129) randomised study will be addressing the role of QFR-guided revascularisation in patients undergoing primary valve surgery.
Limitations
It is important to acknowledge the significant limitations of this study. Our sample size is small and further validation is required across larger patient cohorts. In this study, the QFR calculations were performed offline by a core laboratory and our work would be strengthened through online analysis, which has previously been demonstrated to be feasible with QFR technology (21,23). A significant proportion of vessels (16%) were not suitable for QFR analysis, which may limit the clinical applicability of this technology, although this limitation could potentially be overcome through online analysis. While core laboratory measurements of QFR generally demonstrate good reproducibility (35), the inter- and intra-observer reproducibility of QFR is dependent on observer experience, angiographic quality and coronary artery stenosis severity, and our study would have been strengthened through formal assessment of inter- and intra-observer variability (36). Our study only analysed pre-TAVI indices and would be strengthened through the measurement of post-TAVI values, as QFR has superior diagnostic performance to predict post-TAVI FFR values (28). Our study did not report clinical outcomes and would be strengthened with this information, as low QFR values have been associated with worse clinical outcomes, (25,37-39). Resting full cycle ratio was not assessed in this study, and our study would have been strengthened through addition of this non-hyperaemic index. Computed tomography-derived FFR (CTFFR) has recently been demonstrated to yield reasonable diagnostic accuracy to an FFR reference standard (6) and this study would be strengthened through a comparison of the diagnostic performance of QFR and CTFFR. Our study excluded patients with significant left main coronary artery disease, and further validation is required within this patient cohort.
Conclusions
QFR demonstrates acceptable diagnostic performance when both FFR and non-hyperaemic pressure indices are used as reference standards in patients with severe aortic stenosis. If validated in future larger studies, QFR may be considered as an alternative to both FFR and non-hyperaemic indices for the physiological assessment of coronary lesions in patients with severe aortic stenosis.
Acknowledgments
Funding: Dr. Brown was supported by both a National Health and Medical Research Early Career Fellowship and National Heart Foundation Post-Doctoral Scholarship.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-574/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-574/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-21-574/coif). RG reports personal fees from Boston Scientific, outside the submitted work. LM reports personal fees from Boston Scientific, outside the submitted work. AJB reports personal fees from Abbott Laboratories and Boston Scientific, outside the submitted work. DTLW currently serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from February 2021 to January 2023. The other authors have no conflicts of interest to declare
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was reviewed by a Monash Health Human Research Ethics Committee (HREC/43524/MonH-2018-67705v1) and all participants provided informed consent for inclusion in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lunardi M, Scarsini R, Venturi G, et al. Physiological Versus Angiographic Guidance for Myocardial Revascularization in Patients Undergoing Transcatheter Aortic Valve Implantation. J Am Heart Assoc 2019;8:e012618. [Crossref] [PubMed]
- Duchnowski P, Szymański P, Kuśmierczyk M, et al. Usefulness of FRAIL Scale in Heart Valve Diseases. Clin Interv Aging 2020;15:1071-5. [Crossref] [PubMed]
- Ahmad Y, Götberg M, Cook C, et al. Coronary Hemodynamics in Patients With Severe Aortic Stenosis and Coronary Artery Disease Undergoing Transcatheter Aortic Valve Replacement: Implications for Clinical Indices of Coronary Stenosis Severity. JACC Cardiovasc Interv 2018;11:2019-31. [Crossref] [PubMed]
- Scarsini R, Pesarini G, Zivelonghi C, et al. Physiologic evaluation of coronary lesions using instantaneous wave-free ratio (iFR) in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention 2018;13:1512-9. [Crossref] [PubMed]
- Westra J. TuS. Overview of Quantitative Flow Ratio and Optical Flow Ratio in the Assessment of Intermediate Coronary Lesions. US Cardiology Review 2020;14:e09. [Crossref]
- Michail M, Ihdayhid AR, Comella A, et al. Feasibility and Validity of Computed Tomography-Derived Fractional Flow Reserve in Patients With Severe Aortic Stenosis: The CAST-FFR Study. Circ Cardiovasc Interv 2021;14:e009586. [Crossref] [PubMed]
- Comella A, Michail M, Chan J, et al. Discordance Between Hyperemia and Nonhyperemia Pressure Indexes in Patients With Severe Aortic Stenosis. JACC Cardiovasc Interv 2021;14:356-9. [Crossref] [PubMed]
- Mehta OH, Hay M, Lim RY, et al. Comparison of diagnostic performance between quantitative flow ratio, non-hyperemic pressure indices and fractional flow reserve. Cardiovasc Diagn Ther 2020;10:442-52. [Crossref] [PubMed]
- Faroux L, Guimaraes L, Wintzer-Wehekind J, et al. Coronary Artery Disease and Transcatheter Aortic Valve Replacement: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;74:362-72. [Crossref] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35-71. [Crossref] [PubMed]
- Witberg G, Regev E, Chen S, et al. The Prognostic Effects of Coronary Disease Severity and Completeness of Revascularization on Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2017;10:1428-35. [Crossref] [PubMed]
- Redwood S. The percutaneous coronary intervention prior to transcatheter aortic valve implantation trial: ACTIVATION. Presented at: PCR Valves 2020. November 22, 2020.
- Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503-16. [Crossref] [PubMed]
- Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213-24. [Crossref] [PubMed]
- De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991-1001. [Crossref] [PubMed]
- Davies JE, Sen S, Dehbi HM, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med 2017;376:1824-34. [Crossref] [PubMed]
- Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med 2017;376:1813-23. [Crossref] [PubMed]
- Kleczynski P, Dziewierz A, Rzeszutko L, et al. Hyperemic versus non-hyperemic indexes for coronary physiology assessment in patients with severe aortic stenosis. Adv Med Sci 2021;66:366-71. [Crossref] [PubMed]
- Scarsini R, Lunardi M, Venturi G, et al. Long-term variations of FFR and iFR after transcatheter aortic valve implantation. Int J Cardiol 2020;317:37-41. [Crossref] [PubMed]
- Tu S, Westra J, Yang J, et al. Diagnostic Accuracy of Fast Computational Approaches to Derive Fractional Flow Reserve From Diagnostic Coronary Angiography: The International Multicenter FAVOR Pilot Study. JACC Cardiovasc Interv 2016;9:2024-35. [Crossref] [PubMed]
- Westra J, Andersen BK, Campo G, et al. Diagnostic Performance of In-Procedure Angiography-Derived Quantitative Flow Reserve Compared to Pressure-Derived Fractional Flow Reserve: The FAVOR II Europe-Japan Study. J Am Heart Assoc 2018;7:009603. [Crossref] [PubMed]
- Westra J, Tu S, Winther S, et al. Evaluation of Coronary Artery Stenosis by Quantitative Flow Ratio During Invasive Coronary Angiography: The WIFI II Study (Wire-Free Functional Imaging II). Circ Cardiovasc Imaging 2018;11:e007107. [Crossref] [PubMed]
- Xu B, Tu S, Qiao S, et al. Diagnostic Accuracy of Angiography-Based Quantitative Flow Ratio Measurements for Online Assessment of Coronary Stenosis. J Am Coll Cardiol 2017;70:3077-87. [Crossref] [PubMed]
- Lee KY, Hwang BH, Kim MJ, et al. Influence of lesion and disease subsets on the diagnostic performance of the quantitative flow ratio in real-world patients. Sci Rep 2021;11:2995. [Crossref] [PubMed]
- Spitaleri G, Tebaldi M, Biscaglia S, et al. Quantitative Flow Ratio Identifies Nonculprit Coronary Lesions Requiring Revascularization in Patients With ST-Segment-Elevation Myocardial Infarction and Multivessel Disease. Circ Cardiovasc Interv 2018;11:e006023. [Crossref] [PubMed]
- Tebaldi M, Biscaglia S, Erriquez A, et al. Comparison of quantitative flow ratio, Pd/Pa and diastolic hyperemia-free ratio versus fractional flow reserve in non-culprit lesion of patients with non ST-segment elevation myocardial infarction. Catheter Cardiovasc Interv 2021;98:1057-65. [Crossref] [PubMed]
- Mejía-Rentería H, Nombela-Franco L, Paradis JM, et al. Angiography-based quantitative flow ratio versus fractional flow reserve in patients with coronary artery disease and severe aortic stenosis. EuroIntervention 2020;16:e285-92. [Crossref] [PubMed]
- Sejr-Hansen M, Christiansen EH, Ahmad Y, et al. Performance of quantitative flow ratio in patients with aortic stenosis undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2022;99:68-73. [Crossref] [PubMed]
- Michail M, Davies JE, Cameron JD, et al. Pathophysiological coronary and microcirculatory flow alterations in aortic stenosis. Nat Rev Cardiol 2018;15:420-31. [Crossref] [PubMed]
- De Bruyne B, Baudhuin T, Melin JA, et al. Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation 1994;89:1013-22. [Crossref] [PubMed]
- Scarsini R, Cantone R, Venturi G, et al. Correlation between intracoronary physiology and myocardial perfusion imaging in patients with severe aortic stenosis. Int J Cardiol 2019;292:162-5. [Crossref] [PubMed]
- Kleczynski P, Dziewierz A, Rzeszutko L, et al. Quantitative flow ratio for evaluation of borderline coronary lesions in patients with severe aortic stenosis. Rev Esp Cardiol (Engl Ed) 2021. [Epub ahead of print].
- Scarsini R, Pesarini G, Lunardi M, et al. Observations from a real-time, iFR-FFR "hybrid approach" in patients with severe aortic stenosis and coronary artery disease undergoing TAVI. Cardiovasc Revasc Med 2018;19:355-9. [Crossref] [PubMed]
- Park SJ, Kang SJ, Ahn JM, et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC Cardiovasc Interv 2012;5:1029-36. [Crossref] [PubMed]
- Chang Y, Chen L, Westra J, et al. Reproducibility of quantitative flow ratio: An inter-core laboratory variability study. Cardiol J 2020;27:230-7. [Crossref] [PubMed]
- Westra J, Sejr-Hansen M, Koltowski L, et al. Reproducibility of quantitative flow ratio: the QREP study. EuroIntervention 2022;17:1252-9. [Crossref] [PubMed]
- Bär S, Kavaliauskaite R, Ueki Y, et al. Quantitative Flow Ratio to Predict Nontarget Vessel-Related Events at 5 Years in Patients With ST-Segment-Elevation Myocardial Infarction Undergoing Angiography-Guided Revascularization. J Am Heart Assoc 2021;10:e019052. [Crossref] [PubMed]
- Tang J, Lai Y, Tu S, et al. Quantitative flow ratio-guided residual functional SYNTAX score for risk assessment in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. EuroIntervention 2021;17:e287-93. [Crossref] [PubMed]
- Biscaglia S, Tebaldi M, Brugaletta S, et al. Prognostic Value of QFR Measured Immediately After Successful Stent Implantation: The International Multicenter Prospective HAWKEYE Study. JACC Cardiovasc Interv 2019;12:2079-88. [Crossref] [PubMed]


